Functional characterization of peroxisome proliferator-activated receptor-β/δ expression in colon cancer
- PMID: 21400612
- PMCID: PMC3482838
- DOI: 10.1002/mc.20757
Functional characterization of peroxisome proliferator-activated receptor-β/δ expression in colon cancer
Abstract
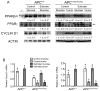
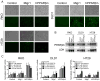
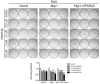
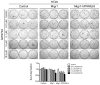
This study critically examined the role of PPARβ/δ in colon cancer models. Expression of PPARβ/δ mRNA and protein was lower and expression of CYCLIN D1 protein higher in human colon adenocarcinomas compared to matched non-transformed tissue. Similar results were observed in colon tumors from Apc(+/Min-FCCC) mice compared to control tissue. Dietary administration of sulindac to Apc(+/Min-FCCC) mice had no influence on expression of PPARβ/δ in normal colon tissue or colon tumors. Cleaved poly (ADP-ribose) polymerase (PARP) was either increased or unchanged, while expression of 14-3-3ε was not influenced in human colon cancer cell lines cultured with the PPARβ/δ ligand GW0742 under conditions known to increase apoptosis. While DLD1 cells exhibited fewer early apoptotic cells after ligand activation of PPARβ/δ following treatment with hydrogen peroxide, this change was associated with an increase in late apoptotic/necrotic cells, but not an increase in viable cells. Stable over-expression of PPARβ/δ in human colon cancer cell lines enhanced ligand activation of PPARβ/δ and inhibition of clonogenicity in HT29 cells. These studies are the most quantitative to date to demonstrate that expression of PPARβ/δ is lower in human and Apc(+/Min-FCCC) mouse colon tumors than in corresponding normal tissue, consistent with the finding that increasing expression and activation of PPARβ/δ in human colon cancer cell lines inhibits clonogenicity. Because ligand-induced attenuation of early apoptosis can be associated with more late, apoptotic/necrotic cells, but not more viable cells, these studies illustrate why more comprehensive analysis of PPARβ/δ-dependent modulation of apoptosis is required in the future.
Copyright © 2011 Wiley Periodicals, Inc.
Figures




References
-
- Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-β/δ in epithelial cell growth and differentiation. Cell Signal. 2006;18(1):9–20. - PubMed
-
- Berglund L, Bjorling E, Oksvold P, et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7(10):2019–2027. - PubMed
-
- Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology. 2001;142(10):4195–4202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA124533/CA/NCI NIH HHS/United States
- CA97999/CA/NCI NIH HHS/United States
- R01 CA140487/CA/NCI NIH HHS/United States
- N01 CN043309/CA/NCI NIH HHS/United States
- R01 CA140369/CA/NCI NIH HHS/United States
- R01 CA141029/CA/NCI NIH HHS/United States
- CA006927/CA/NCI NIH HHS/United States
- CA140487/CA/NCI NIH HHS/United States
- CA 140369/CA/NCI NIH HHS/United States
- CA141029/CA/NCI NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
- CA124533/CA/NCI NIH HHS/United States
- CA126826/CA/NCI NIH HHS/United States
- R21 CA129467/CA/NCI NIH HHS/United States
- R01 CA097999/CA/NCI NIH HHS/United States
- R01 CA126826/CA/NCI NIH HHS/United States
- CA129467/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

