Determining the enantioselectivity of chiral catalysts by mass spectrometric screening of their racemic forms
- PMID: 21401128
- PMCID: PMC3068606
- DOI: 10.1021/ja111700e
Determining the enantioselectivity of chiral catalysts by mass spectrometric screening of their racemic forms
Abstract
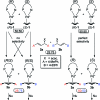
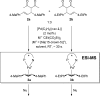
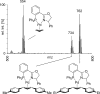
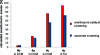
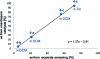
The enantioselectivity of a chiral catalyst can be determined from its racemic form by mass spectrometric screening of a nonequal mixture of two mass-labeled quasienantiomeric substrates. The presented method opens up new possibilities for evaluating catalyst structures that are not readily available in enantiomerically pure form.
© 2011 American Chemical Society
Figures


References
-
-
For reviews, see:
- Reetz M. T. Angew. Chem. 2008, 120, 2592.
- Angew. Chem., Int. Ed. 2008, 47, 2556. - PubMed
- de Vries J. G.; de Vries A. H. M. Eur. J. Org. Chem. 2003, 799.
- Hagemeyer A.; Jandeleit B.; Liu Y.; Poojary D. M.; Turner H. W.; Volpe A. F.; Weinberg W. H. Appl. Catal., A 2001, 221, 23.
-
-
- Lagasse F.; Tsukamoto M.; Welch C. J.; Kagan H. B. J. Am. Chem. Soc. 2003, 125, 7490. - PubMed
-
- Blackmond D. G.; Hodnett N. S.; Lloyd-Jones G. C. J. Am. Chem. Soc. 2006, 128, 7450. - PubMed
-
- Markert C.; Pfaltz A. Angew. Chem. 2004, 116, 2552. - PubMed
- Angew. Chem., Int. Ed. 2004, 43, 2498. - PubMed
- Markert C.; Rösel P.; Pfaltz A. J. Am. Chem. Soc. 2008, 130, 3234. - PubMed
- Müller C. A.; Pfaltz A. Angew. Chem. 2008, 120, 3411. - PubMed
- Angew. Chem., Int. Ed. 2008, 47, 3363. - PubMed
- Teichert A.; Pfaltz A. Angew. Chem. 2008, 120, 3408. - PubMed
- Angew. Chem., Int. Ed. 2008, 47, 3360. - PubMed
- Fleischer I.; Pfaltz A. Chem.—Eur. J. 2010, 16, 95. - PubMed
- For a review, see: Müller C. A.; Markert C.; Teichert A. M.; Pfaltz A. Chem. Commun. 2009, 1607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

