Lessons from seven decades of antituberculosis drug discovery
- PMID: 21401509
- PMCID: PMC6380367
- DOI: 10.2174/156802611795429158
Lessons from seven decades of antituberculosis drug discovery
Abstract
Despite massive global efforts tuberculosis rates continue to climb and drug-resistance rates are rising to alarming levels. Discovering new agents for treating this bacterial pathogen poses unique challenges, but these challenges have been faced throughout the entire modern history of research into anti-infectives. This review looks back at every decade since the 1940s and summarizes the most important drugs developed during each decade highlighting the lessons learned during these successful medicinal chemistry programs. Looking forward we must accelerate the integration of these past lessons with the impressive advances that have been made in the basic understanding of the biology of this disease.
Figures
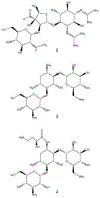
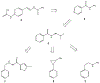


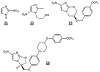
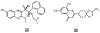
References
-
- Donoghue HD; Spigelman M; Greenblatt CL; Lev-Maor G; Bar-Gal GK; Matheson C; Vernon K; Nerlich AG; Zink AR Tuberculosis: from prehistory to Robert Koch, as revealed by ancient DNA. Lancet Infect. Dis, 2004, 4, 584–592. - PubMed
-
- Glaziou P; Floyd K; Raviglione M Global burden and epidemiology of tuberculosis. Clin. Chest Med, 2009, 30, 621–36, vii. - PubMed
-
- Aziz MA; Wright A; Laszlo A; De Muynck A; Portaels F; Van Deun A; Wells C; Nunn P; Blanc L; Raviglione M Epidemiology of anti-tuberculosis drug resistance (the Global Project on Antituberculosis Drug Resistance Surveillance): an updated analysis. Lancet, 2006, 368, 2142–2154. - PubMed
-
- Riccardi G; Pasca MR; Buroni S Mycobacterium tuberculosis: drug resistance and future perspectives. Future Microbiol, 2009, 4, 597–614. - PubMed
-
- Wright A; Zignol M; Van Deun A; Falzon D; Gerdes SR; Feldman K; Hoffner S; Drobniewski F; Barrera L; van Soolingen D; Boulabhal F; Paramasivan CN; Kam KM; Mitarai S; Nunn P; Raviglione M Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet, 2009, 373, 1861–1873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

