aprABC: a Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome
- PMID: 21401735
- PMCID: PMC3138066
- DOI: 10.1111/j.1365-2958.2011.07601.x
aprABC: a Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome
Abstract
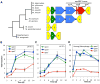
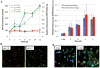
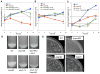
Following phagocytosis by macrophages, Mycobacterium tuberculosis (Mtb) senses the intracellular environment and remodels its gene expression for growth in the phagosome. We have identified an acid and phagosome regulated (aprABC) locus that is unique to the Mtb complex and whose gene expression is induced during growth in acidic environments in vitro and in macrophages. Using the aprA promoter, we generated a strain that exhibits high levels of inducible fluorescence in response to growth in acidic medium in vitro and in macrophages. aprABC expression is dependent on the two-component regulator phoPR, linking phoPR signalling to pH sensing. Deletion of the aprABC locus causes defects in gene expression that impact aggregation, intracellular growth, and the relative levels of storage and cell wall lipids. We propose a model where phoPR senses the acidic pH of the phagosome and induces aprABC expression to fine-tune processes unique for intracellular adaptation of Mtb complex bacteria.
© 2011 Blackwell Publishing Ltd.
Figures
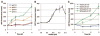


References
-
- Asensio JG, Maia C, Ferrer NL, Barilone N, Laval F, Soto CY, et al. The virulence-associated two component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem. 2006;281:1313–1316. - PubMed
-
- Basu S, Pathak SK, Banerjee A, Pathak S, Bhattacharyya A, Yang ZH, et al. Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by toll-like receptor 2-dependent release of tumor necrosis factor-alpha. J Biol Chem. 2007;282:1039–1050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

