B cells Can Modulate the CD8 Memory T Cell after DNA Vaccination Against Experimental Tuberculosis
- PMID: 21401938
- PMCID: PMC3066104
- DOI: 10.1186/1479-0556-9-5
B cells Can Modulate the CD8 Memory T Cell after DNA Vaccination Against Experimental Tuberculosis
Abstract
Background: Although B cells are important as antigen presenting cells (APC) during the immune response, their role in DNA vaccination models is unknown.
Methods: In this study in vitro and in vivo experiments were performed to evaluate the ability of B cells to protect mice against Mycobacterium tuberculosis challenge.
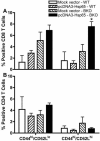
Results: In vitro and in vivo studies showed that B cells efficiently present antigens after naked plasmid pcDNA3 encoding M. leprae 65-kDa heat shock protein (pcDNA3-Hsp65) internalization and protect B knock-out (BKO) mice against Mycobacterium tuberculosis infection. pcDNA3-Hsp65-transfected B cells adoptively transferred into BKO mice rescued the memory phenotypes and reduced the number of CFU compared to wild-type mice.
Conclusions: These data not only suggest that B cells play an important role in the induction of CD8 T cells but also that they improve bacterial clearance in DNA vaccine model.
Figures
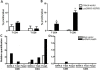

References
LinkOut - more resources
Full Text Sources
Research Materials

