Uptake of newer methodological developments and the deployment of meta-analysis in diagnostic test research: a systematic review
- PMID: 21401947
- PMCID: PMC3065444
- DOI: 10.1186/1471-2288-11-27
Uptake of newer methodological developments and the deployment of meta-analysis in diagnostic test research: a systematic review
Abstract
Background: The last decade has seen a number of methodological developments in meta-analysis of diagnostic test studies. However, it is unclear whether such developments have permeated the wider research community and on which applications they are being deployed. The objective was to assess the uptake and deployment of the main methodological developments in the meta-analysis of diagnostic tests, and identify the tests and target disorders most commonly evaluated by meta-analysis.
Methods: Design--systematic review. Data Sources--Medline, EMBASE, CINAHL, Cochrane, PsychInfo, Global health, HMIC, and AMED were searched for studies published before 31st December 2008. Selection criteria--studies were included if they satisfied all of the following: evaluated a diagnostic test; measured test performance; searched two or more databases; stated search terms and inclusion criteria; used a statistical method to summarise performance. Data extraction--included the following data items: year; test; reference standard; target disorder; setting; statistical and quality methods.
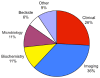
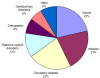
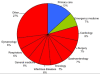
Results: 236 studies were included. Over the last 5 years the number of meta-analyses published has increased, but the uptake of new statistical methods lags behind. Pooling the sensitivity and specificity and using the SROC remain the preferred methods for analysis in 70% of studies, with the bivariate random effects and HSROC model being used in only 22% and 5% of studies respectively. In contrast, between 2006 and 2008 the QUADAS tool was used in 40% of studies. Broadly, radiological imaging was the most frequent category of tests analysed (36%), with cancer (22%) and infection (21%) being the most common categories of target disorder. Nearly 80% of tests analysed were those normally used in specialist settings.
Conclusion: Although quality assessment in meta-analyses has improved with the introduction of QUADAS, uptake of the newer statistical methods is still lagging behind. Furthermore, the focus of secondary research seems to be in evaluating specialist tests in specialist settings, in contrast to the more routine tests and settings encountered in the majority of clinical practice.
Figures


References
-
- Knottnerus JA, van Weel C, Muris JWM. In: The Evidence Base of Clinical Diagnosis. Knottnerus JA, editor. London: BMJ Books; 2002. General introduction: Evaluation of diagnostic procedures; pp. 1–17.
-
- Tatsioni A, Zarin DA, Aronson N, Samson DJ, Flamm CR, Schmid C, Lau J. Challenges in Systematic Reviews of Diagnostic Technologies. Ann Intern Med. 2005;142:1048–1055. - PubMed
-
- Knottnerus JA, Muris JWM. In: The Evidence Base of Clinical Diagnosis. Knottnerus JA, editor. London: BMJ Books; 2002. Assessment of the accuracy of diagnostic tests: the cross-sectional study; pp. 39–59.
-
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–4. doi: 10.1136/bmj.326.7379.41. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

