Acetaminophen-cysteine adducts during therapeutic dosing and following overdose
- PMID: 21401949
- PMCID: PMC3066114
- DOI: 10.1186/1471-230X-11-20
Acetaminophen-cysteine adducts during therapeutic dosing and following overdose
Abstract
Background: Acetaminophen-cysteine adducts (APAP-CYS) are a specific biomarker of acetaminophen exposure. APAP-CYS concentrations have been described in the setting of acute overdose, and a concentration >1.1 nmol/ml has been suggested as a marker of hepatic injury from acetaminophen overdose in patients with an ALT >1000 IU/L. However, the concentrations of APAP-CYS during therapeutic dosing, in cases of acetaminophen toxicity from repeated dosing and in cases of hepatic injury from non-acetaminophen hepatotoxins have not been well characterized. The objective of this study is to describe APAP-CYS concentrations in these clinical settings as well as to further characterize the concentrations observed following acetaminophen overdose.
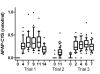
Methods: Samples were collected during three clinical trials in which subjects received 4 g/day of acetaminophen and during an observational study of acetaminophen overdose patients. Trial 1 consisted of non-drinkers who received APAP for 10 days, Trial 2 consisted of moderate drinkers dosed for 10 days and Trial 3 included subjects who chronically abuse alcohol dosed for 5 days. Patients in the observational study were categorized by type of acetaminophen exposure (single or repeated). Serum APAP-CYS was measured using high pressure liquid chromatography with electrochemical detection.
Results: Trial 1 included 144 samples from 24 subjects; Trial 2 included 182 samples from 91 subjects and Trial 3 included 200 samples from 40 subjects. In addition, we collected samples from 19 subjects with acute acetaminophen ingestion, 7 subjects with repeated acetaminophen exposure and 4 subjects who ingested another hepatotoxin. The mean (SD) peak APAP-CYS concentrations for the Trials were: Trial 1- 0.4 (0.20) nmol/ml, Trial 2- 0.1 (0.09) nmol/ml and Trial 3- 0.3 (0.12) nmol/ml. APAP-CYS concentrations varied substantially among the patients with acetaminophen toxicity (0.10 to 27.3 nmol/ml). No subject had detectable APAP-CYS following exposure to a non-acetaminophen hepatotoxin.
Conclusions: Lower concentrations of APAP-CYS are detectable after exposure to therapeutic doses of acetaminophen and higher concentrations are detected after acute acetaminophen overdose and in patients with acetaminophen toxicity following repeated exposure.
Figures


References
-
- Pumford NR, Hinson JA, Potter DW, Rowland KL, Benson RW, Roberts DW. Immunochemical quantitation of 3-(cystein-S-yl)acetaminophen adducts in serum and liver proteins of acetaminophen-treated mice. J Pharmacol Exp Ther. 1989;248(1):190–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

