Biophysical coarse-grained modeling provides insights into transport through the nuclear pore complex
- PMID: 21402022
- PMCID: PMC3059569
- DOI: 10.1016/j.bpj.2011.01.061
Biophysical coarse-grained modeling provides insights into transport through the nuclear pore complex
Abstract
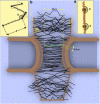
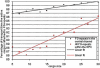
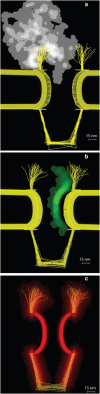
The nuclear pore complex (NPC) is the gatekeeper of the nucleus, capable of actively discriminating between the active and inert cargo while accommodating a high rate of translocations. The biophysical mechanisms underlying transport, however, remain unclear due to the lack of information about biophysical factors playing role in transport. Based on published experimental data, we have established a coarse-grained model of an intact NPC structure to examine nucleocytoplasmic transport with refined spatial and temporal resolutions. Using our model, we estimate the transport time versus cargo sizes. Our findings suggest that the mean transport time of cargos smaller than 15 nm is independent of size, while beyond this size, there is a sharp increase in the mean transport time. The model confirms that kap-FG hydrophobicity is sufficient for active cargo transport. Moreover, our model predicts that during translocation, small and large cargo-complexes are hydrophobically attached to FG-repeat domains for 86 and 96% of their transport time, respectively. Inside the central channel FG-repeats form a thick layer on the wall leaving an open tube. The cargo-complex is almost always attached to this layer and diffuses back and forth, regardless of the cargo size. Finally, we propose a plausible model for transport in which the NPC can be viewed as a lubricated gate. This model incorporates basic assumptions underlying virtual-gate and reduction-of-dimensionality models with the addition of the FG-layer inside the central channel acting as a lubricant.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Brownian dynamics simulation of nucleocytoplasmic transport: a coarse-grained model for the functional state of the nuclear pore complex.PLoS Comput Biol. 2011 Jun;7(6):e1002049. doi: 10.1371/journal.pcbi.1002049. Epub 2011 Jun 2. PLoS Comput Biol. 2011. PMID: 21673865 Free PMC article.
-
Emergence of selectivity and specificity in a coarse-grained model of the nuclear pore complex with sequence-agnostic FG-Nups.Phys Chem Chem Phys. 2023 Dec 13;25(48):32824-32836. doi: 10.1039/d3cp03746k. Phys Chem Chem Phys. 2023. PMID: 38018404
-
Charge of karyopherins and nuclear FG-Nups are key ingredients of nucleocytoplasmic transport.Biophys J. 2025 Jan 21;124(2):215-226. doi: 10.1016/j.bpj.2024.11.3313. Epub 2024 Nov 26. Biophys J. 2025. PMID: 39600095
-
Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality.Traffic. 2005 May;6(5):421-7. doi: 10.1111/j.1600-0854.2005.00287.x. Traffic. 2005. PMID: 15813752 Review.
-
How to operate a nuclear pore complex by Kap-centric control.Nucleus. 2015;6(5):366-72. doi: 10.1080/19491034.2015.1090061. Nucleus. 2015. PMID: 26338152 Free PMC article. Review.
Cited by
-
A physical model describing the interaction of nuclear transport receptors with FG nucleoporin domain assemblies.Elife. 2016 Apr 8;5:e14119. doi: 10.7554/eLife.14119. Elife. 2016. PMID: 27058170 Free PMC article.
-
Regulating transport efficiency through the nuclear pore complex: The role of binding affinity with FG-Nups.Mol Biol Cell. 2024 Dec 1;35(12):ar149. doi: 10.1091/mbc.E24-05-0224. Epub 2024 Oct 30. Mol Biol Cell. 2024. PMID: 39475712 Free PMC article.
-
Nuclear pore complex protein sequences determine overall copolymer brush structure and function.Biophys J. 2014 May 6;106(9):1997-2007. doi: 10.1016/j.bpj.2014.03.021. Biophys J. 2014. PMID: 24806932 Free PMC article.
-
Probing the disordered domain of the nuclear pore complex through coarse-grained molecular dynamics simulations.Biophys J. 2014 Sep 16;107(6):1393-402. doi: 10.1016/j.bpj.2014.07.060. Biophys J. 2014. PMID: 25229147 Free PMC article.
-
Evolutionarily Conserved Sequence Features Regulate the Formation of the FG Network at the Center of the Nuclear Pore Complex.Sci Rep. 2015 Nov 6;5:15795. doi: 10.1038/srep15795. Sci Rep. 2015. PMID: 26541386 Free PMC article.
References
-
- Mooren O.L., Erickson E.S., Dunn R.C. Nuclear side conformational changes in the nuclear pore complex following calcium release from the nuclear membrane. Phys. Biol. 2004;1:125–134. - PubMed
-
- Panté N., Aebi U. Molecular dissection of the nuclear pore complex. Crit. Rev. Biochem. Mol. Biol. 1996;31:153–199. - PubMed
-
- Jamali T., Jamali Y., Mofrad M.R.K. Nuclear pore complex: biochemistry and biophysics of nucleocytoplasmic transport in health and disease. Intl. Rev. Cell Mol. Biol. 2011;287:233–280. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

