Vibrational motions associated with primary processes in bacteriorhodopsin studied by coherent infrared emission spectroscopy
- PMID: 21402041
- PMCID: PMC3059580
- DOI: 10.1016/j.bpj.2011.02.011
Vibrational motions associated with primary processes in bacteriorhodopsin studied by coherent infrared emission spectroscopy
Abstract
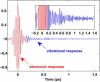
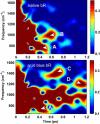
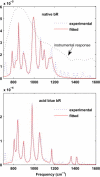
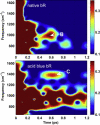
The primary energetic processes driving the functional proton pump of bacteriorhodopsin take place in the form of complex molecular dynamic events after excitation of the retinal chromophore into the Franck-Condon state. These early events include a strong electronic polarization, skeletal stretching, and all-trans-to-13-cis isomerization upon formation of the J intermediate. The effectiveness of the photoreaction is ensured by a conical intersection between the electronic excited and ground states, providing highly nonadiabatic coupling to nuclear motions. Here, we study real-time vibrational coherences associated with these motions by analyzing light-induced infrared emission from oriented purple membranes in the 750-1400 cm(-)(1) region. The experimental technique applied is based on second-order femtosecond difference frequency generation on macroscopically ordered samples that also yield information on phase and direction of the underlying motions. Concerted use of several analysis methods resulted in the isolation and characterization of seven different vibrational modes, assigned as C-C stretches, out-of-plane methyl rocks, and hydrogen out-of-plane wags, whereas no in-plane H rock was found. Based on their lifetimes and several other criteria, we deduce that the majority of the observed modes take place on the potential energy surface of the excited electronic state. In particular, the direction sensitivity provides experimental evidence for large intermediate distortions of the retinal plane during the excited-state isomerization process.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Spudich J.L., Yang C.S., Spudich E.N. Retinylidene proteins: structures and functions from archaea to humans. Annu. Rev. Cell Dev. Biol. 2000;16:365–392. - PubMed
-
- Béjà O., Aravind L., DeLong E.F. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science. 2000;289:1902–1906. - PubMed
-
- Haupts U., Tittor J., Oesterhelt D. Closing in on bacteriorhodopsin: progress in understanding the molecule. Annu. Rev. Biophys. Biomol. Struct. 1999;28:367–399. - PubMed
-
- Lanyi J.K. Bacteriorhodopsin. Annu. Rev. Physiol. 2004;66:665–688. - PubMed
-
- Aharoni A., Hou B., Zhong Q. Non-isomerizable artificial pigments: implications for the primary light-induced events in bacteriorhodopsin. Biochemistry (Mosc.) 2001;66:1210–1219. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

