Immune challenge activates neural inputs to the ventrolateral bed nucleus of the stria terminalis
- PMID: 21402087
- PMCID: PMC3118915
- DOI: 10.1016/j.physbeh.2011.03.006
Immune challenge activates neural inputs to the ventrolateral bed nucleus of the stria terminalis
Abstract
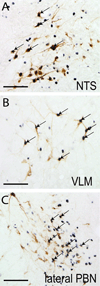
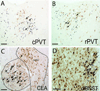
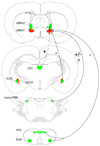
Hypothalamo-pituitary-adrenal (HPA) axis activation in response to infection is an important mechanism by which the nervous system can suppress inflammation. HPA output is controlled by the hypothalamic paraventricular nucleus (PVN). Previously, we determined that noradrenergic inputs to the PVN contribute to, but do not entirely account for, the ability of bacterial endotoxin (i.e., lipopolysacharide, LPS) to activate the HPA axis. The present study investigated LPS-induced recruitment of neural inputs to the ventrolateral bed nucleus of the stria terminalis (vlBNST). GABAergic projections from the vlBNST inhibit PVN neurons at the apex of the HPA axis; thus, we hypothesize that LPS treatment activates inhibitory inputs to the vlBNST to thereby "disinhibit" the PVN and increase HPA output. To test this hypothesis, retrograde neural tracer was iontophoretically delivered into the vlBNST of adult male rats to retrogradely label central sources of axonal input. After one week, rats were injected i.p. with either LPS (200 μg/kg BW) or saline vehicle, and then perfused with fixative 2.5h later. Brains were processed for immunohistochemical localization of retrograde tracer and the immediate-early gene product, Fos (a marker of neural activation). Brain regions that provide inhibitory input to the vlBNST (e.g., caudal nucleus of the solitary tract, central amygdala, dorsolateral BNST) were preferentially activated by LPS, whereas sources of excitatory input (e.g., paraventricular thalamus, medial prefrontal cortex) were not activated or were activated less robustly. These results suggest that LPS treatment recruits central neural systems that actively suppress vlBNST neural activity, thereby removing a potent source of inhibitory control over the HPA axis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Buller KM. Circumventricular organs: gateways to the brain role of circumventricular organs in pro-inflammatory cytokine-induced activation of the hypothalamic- pituitary-adrenal axis. Clinical and Experimental Pharmacology and Physiology. 2001;28:581–589. - PubMed
-
- Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Molecular Psychiatry. 2000;5:604–615. - PubMed
-
- Goehler LE, Gaykema RPA. Neural pathways mediating behavioral changes associated with immunological challenge. In: Siegal A, Zalcman S, editors. The Neuroimmunological Basis of Behavior and Mental Disorders. 1st ed. New York: Springer; 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

