Alveolar macrophages initiate the systemic microvascular inflammatory response to alveolar hypoxia
- PMID: 21402178
- PMCID: PMC3155636
- DOI: 10.1016/j.resp.2011.03.008
Alveolar macrophages initiate the systemic microvascular inflammatory response to alveolar hypoxia
Abstract
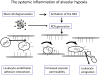
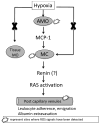
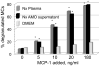
Alveolar hypoxia occurs as a result of a decrease in the environmental [Formula: see text] , as in altitude, or in clinical conditions associated with a global or regional decrease in alveolar ventilation. Systemic effects, in most of which an inflammatory component has been identified, frequently accompany both acute and chronic forms of alveolar hypoxia. Experimentally, it has been shown that acute exposure to environmental hypoxia causes a widespread systemic inflammatory response in rats and mice. Recent research has demonstrated that alveolar macrophages, in addition to their well known intrapulmonary functions, have systemic, extrapulmonary effects when activated, and indirect evidence suggest these cells may play a role in the systemic consequences of alveolar hypoxia. This article reviews studies showing that the systemic inflammation of acute alveolar hypoxia observed in rats is not initiated by the low systemic tissue [Formula: see text] , but rather by a chemokine, Monocyte Chemoattractant Protein-1 (MCP-1, or CCL2) released by alveolar macrophages stimulated by hypoxia and transported by the circulation. Circulating MCP-1, in turn, activates perivascular mast cells to initiate the microvascular inflammatory cascade. The research reviewed here highlights the extrapulmonary effects of alveolar macrophages and provides a possible mechanism for some of the systemic effects of alveolar hypoxia.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures





Similar articles
-
Monocyte chemoattractant protein-1 released from alveolar macrophages mediates the systemic inflammation of acute alveolar hypoxia.Am J Respir Cell Mol Biol. 2011 Jul;45(1):53-61. doi: 10.1165/rcmb.2010-0264OC. Epub 2010 Sep 2. Am J Respir Cell Mol Biol. 2011. PMID: 20813992 Free PMC article.
-
The systemic inflammation of alveolar hypoxia is initiated by alveolar macrophage-borne mediator(s).Am J Respir Cell Mol Biol. 2009 Nov;41(5):573-82. doi: 10.1165/rcmb.2008-0417OC. Epub 2009 Feb 24. Am J Respir Cell Mol Biol. 2009. PMID: 19244200 Free PMC article.
-
Alveolar hypoxia, alveolar macrophages, and systemic inflammation.Respir Res. 2009 Jun 22;10(1):54. doi: 10.1186/1465-9921-10-54. Respir Res. 2009. PMID: 19545431 Free PMC article. Review.
-
Dexamethasone blocks the systemic inflammation of alveolar hypoxia at several sites in the inflammatory cascade.Am J Physiol Heart Circ Physiol. 2012 Jul 15;303(2):H168-77. doi: 10.1152/ajpheart.00106.2012. Epub 2012 May 18. Am J Physiol Heart Circ Physiol. 2012. PMID: 22610172 Free PMC article.
-
Alveolar Hypoxia-Induced Pulmonary Inflammation: From Local Initiation to Secondary Promotion by Activated Systemic Inflammation.J Vasc Res. 2016;53(5-6):317-329. doi: 10.1159/000452800. Epub 2016 Dec 15. J Vasc Res. 2016. PMID: 27974708 Review.
Cited by
-
Ubiquinol decreases hemorrhagic shock/resuscitation-induced microvascular inflammation in rat mesenteric microcirculation.Physiol Rep. 2014 Nov 20;2(11):e12199. doi: 10.14814/phy2.12199. Print 2014 Nov 1. Physiol Rep. 2014. PMID: 25413319 Free PMC article.
-
Acclimatization of the systemic microcirculation to alveolar hypoxia is mediated by an iNOS-dependent increase in nitric oxide availability.J Appl Physiol (1985). 2017 Oct 1;123(4):974-982. doi: 10.1152/japplphysiol.00322.2016. Epub 2017 Mar 16. J Appl Physiol (1985). 2017. PMID: 28302706 Free PMC article.
-
The impact of inflammation on respiratory plasticity.Exp Neurol. 2017 Jan;287(Pt 2):243-253. doi: 10.1016/j.expneurol.2016.07.022. Epub 2016 Jul 27. Exp Neurol. 2017. PMID: 27476100 Free PMC article. Review.
-
Distinct hypoxia-induced translational profiles of embryonic and adult-derived macrophages.iScience. 2023 Sep 21;26(12):107985. doi: 10.1016/j.isci.2023.107985. eCollection 2023 Dec 15. iScience. 2023. PMID: 38047075 Free PMC article.
-
Decreased Ambient Oxygen Tension Alters the Expression of Endothelin-1, iNOS and cGMP in Rat Alveolar Macrophages.Int J Med Sci. 2019 Feb 28;16(3):443-449. doi: 10.7150/ijms.28353. eCollection 2019. Int J Med Sci. 2019. PMID: 30911278 Free PMC article.
References
-
- Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361:1967–1974. - PubMed
-
- Beck-Schimmer B, Schimmer RC, Madjdpour C, Bonvini JM, Pasch T, Ward PA. Hypoxia mediates increased neutrophil and macrophage adhesiveness to alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25(6):780–787. - PubMed
-
- Beidleman BA, Muza SR, Fulco CS, Cymerman A, Staab JE, Sawka MN, Lewis SF, Skrinar GS. White blood cell and hormonal responses to 4300 m altitude before and after intermittent altitude exposure. Clin Sci (Lond) 2006;111:163–169. - PubMed
-
- Bonello S, Zahringer C, BelAiba RS, Djordjevic T. Reactive O2 species activate the HIF-1α promoter via a functional NF-κB site. Arterioscler Thromb Vasc Biol; 2007;27:755–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

