Ultrastructural, immunofluorescence, and RNA evidence support the hypothesis of a "new" virus associated with Kawasaki disease
- PMID: 21402552
- PMCID: PMC3068030
- DOI: 10.1093/infdis/jiq136
Ultrastructural, immunofluorescence, and RNA evidence support the hypothesis of a "new" virus associated with Kawasaki disease
Abstract
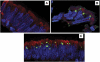
Background: Intracytoplasmic inclusion bodies (ICI) have been identified in ciliated bronchial epithelium of Kawasaki disease (KD) patients using a synthetic antibody derived from acute KD arterial IgA plasma cells; ICI may derive from the KD etiologic agent.
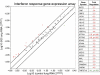
Methods: Acute KD bronchial epithelium was subjected to immunofluorescence for ICI and cytokeratin, high-throughput sequencing, and transmission electron microscopy (TEM). Interferon pathway gene expression profiling was performed on KD lung.
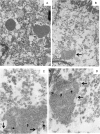
Results: An intermediate filament cytokeratin "cage" was not observed around KD ICI, making it unlikely that ICI are overproduced or misfolded human protein aggregates. Many interferon-stimulated genes were detected in the bronchial epithelium, and significant modulation of the interferon response pathway was observed in the lung tissue of KD patients. No known virus was identified by sequencing. Aggregates of virus-like particles (VLP) were detected by TEM in all 3 acute KD patients from whom nonembedded formalin-fixed lung tissue was available.
Conclusions: KD ICI are most likely virus induced; bronchial cells with ICI contain VLP that share morphologic features among several different RNA viral families. Expedited autopsies and tissue fixation from acute KD fatalities are urgently needed to more clearly ascertain the VLP. These findings are compatible with the hypothesis that the infectious etiologic agent of KD may be a "new" RNA virus.
Figures


References
-
- Rowley AH, Shulman ST, Spike BT, Mask CA, Baker SC. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001;166:1334–43. - PubMed
-
- Rowley AH, Baker SC, Shulman ST, et al. Detection of antigen in bronchial epithelium and macrophages in acute Kawasaki disease by use of synthetic antibody. J Infect Dis. 2004;190:856–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

