Lens fibre cell differentiation and organelle loss: many paths lead to clarity
- PMID: 21402582
- PMCID: PMC3061109
- DOI: 10.1098/rstb.2010.0324
Lens fibre cell differentiation and organelle loss: many paths lead to clarity
Abstract
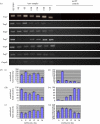
The programmed removal of organelles from differentiating lens fibre cells contributes towards lens transparency through formation of an organelle-free zone (OFZ). Disruptions in OFZ formation are accompanied by the persistence of organelles in lens fibre cells and can contribute towards cataract. A great deal of work has gone into elucidating the nature of the mechanisms and signalling pathways involved. It is apparent that multiple, parallel and redundant pathways are involved in this process and that these pathways form interacting networks. Furthermore, it is possible that the pathways can functionally compensate for each other, for example in mouse knockout studies. This makes sense given the importance of lens clarity in an evolutionary context. Apoptosis signalling and proteolytic pathways have been implicated in both lens fibre cell differentiation and organelle loss, including the Bcl-2 and inhibitor of apoptosis families, tumour necrosis factors, p53 and its regulators (such as Mdm2) and proteolytic enzymes, including caspases, cathepsins, calpains and the ubiquitin-proteasome pathway. Ongoing approaches being used to dissect the molecular pathways involved, such as transgenics, lens-specific gene deletion and zebrafish mutants, are discussed here. Finally, some of the remaining unresolved issues and potential areas for future studies are highlighted.
Figures
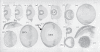
References
-
- Kuszak J. R., Zoltoski R. K., Sivertson C. 2004. Fibre cell organization in crystalline lenses. Exp. Eye Res. 78, 673–68710.1016/j.exer.2003.09.016 (doi:10.1016/j.exer.2003.09.016) - DOI - DOI - PubMed
-
- Bassnett S., Shi Y., Vrensen G. F. J. M. 2011. Biological glass: structural determinants of eye lens transparency. Phil. Trans. R. Soc. B 366, 1250–126410.1098/rstb.2010.0302 (doi:10.1098/rstb.2010.0302) - DOI - DOI - PMC - PubMed
-
- Graw J. 2009. Genetics of crystallins: cataract and beyond. Exp. Eye Res. 88, 173–18910.1016/j.exer.2008.10.011 (doi:10.1016/j.exer.2008.10.011) - DOI - DOI - PubMed
-
- Vrensen G. F., Graw J., De Wolf A. 1991. Nuclear breakdown during terminal differentiation of primary lens fibres in mice: a transmission electron microscopic study. Exp. Eye Res. 52, 647–65910.1016/0014-4835(91)90017-9 (doi:10.1016/0014-4835(91)90017-9) - DOI - DOI - PubMed
-
- Ito M., Yoshioka M. 1999. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat. Embryol. (Berl.) 200, 403–41110.1007/s004290050289 (doi:10.1007/s004290050289) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
