The ageing lens and cataract: a model of normal and pathological ageing
- PMID: 21402586
- PMCID: PMC3061107
- DOI: 10.1098/rstb.2010.0300
The ageing lens and cataract: a model of normal and pathological ageing
Abstract
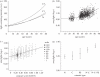
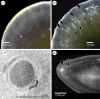
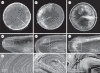
Cataract is a visible opacity in the lens substance, which, when located on the visual axis, leads to visual loss. Age-related cataract is a cause of blindness on a global scale involving genetic and environmental influences. With ageing, lens proteins undergo non-enzymatic, post-translational modification and the accumulation of fluorescent chromophores, increasing susceptibility to oxidation and cross-linking and increased light-scatter. Because the human lens grows throughout life, the lens core is exposed for a longer period to such influences and the risk of oxidative damage increases in the fourth decade when a barrier to the transport of glutathione forms around the lens nucleus. Consequently, as the lens ages, its transparency falls and the nucleus becomes more rigid, resisting the change in shape necessary for accommodation. This is the basis of presbyopia. In some individuals, the steady accumulation of chromophores and complex, insoluble crystallin aggregates in the lens nucleus leads to the formation of a brown nuclear cataract. The process is homogeneous and the affected lens fibres retain their gross morphology. Cortical opacities are due to changes in membrane permeability and enzyme function and shear-stress damage to lens fibres with continued accommodative effort. Unlike nuclear cataract, progression is intermittent, stepwise and non-uniform.
Figures



References
-
- Winkler B. S., Riley M. V. 1991. Relative contributions of epithelial cells and fibers to rabbit lens ATP content and glycolysis. Invest. Ophthalmol. Vis. Sci. 32, 2593–2598 - PubMed
-
- Mathias R. T., White T. W., Gong X. 2010. Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 90, 179–206 10.1152/physrev.00034.2009 (doi:10.1152/physrev.00034.2009) - DOI - PMC - PubMed
-
- Augusteyn R. C. 1981. Protein modification in cataract: possible oxidative mechanism. In Mechanisms of cataract formation in the human lens (ed. Duncan G.), pp. 72–115 New York, NY: Academic Press
-
- Harding J. J. 1991. Cataract: biochemistry, epidemiology and pharmacology. London, UK: Chapman & Hall
-
- Lou M. F. 2003. Redox regulation in the lens. Prog. Retin. Eye Res. 22, 657–682 10.1016/S1350-9462(03)00050-8 (doi:10.1016/S1350-9462(03)00050-8) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
