An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome
- PMID: 21402677
- PMCID: PMC3090243
- DOI: 10.1194/jlr.M014498
An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome
Abstract
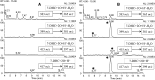
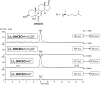
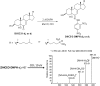
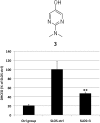
The level of 7-dehydrocholesterol (7-DHC) is elevated in tissues and fluids of Smith-Lemli-Opitz syndrome (SLOS) patients due to defective 7-DHC reductase. Although over a dozen oxysterols have been identified from 7-DHC free radical oxidation in solution, oxysterol profiles in SLOS cells and tissues have never been studied. We report here the identification and complete characterization of a novel oxysterol, 3β,5α-dihydroxycholest-7-en-6-one (DHCEO), as a biomarker for 7-DHC oxidation in fibroblasts from SLOS patients and brain tissue from a SLOS mouse model. Deuterated (d₇)-standards of 7-DHC and DHCEO were synthesized from d₇-cholesterol. The presence of DHCEO in SLOS samples was supported by chemical derivatization in the presence of d₇-DHCEO standard followed by HPLC-MS or GC-MS analysis. Quantification of cholesterol, 7-DHC, and DHCEO was carried out by isotope dilution MS with the d₇-standards. The level of DHCEO was high and correlated well with the level of 7-DHC in all samples examined (R = 0.9851). Based on our in vitro studies in two different cell lines, the mechanism of formation of DHCEO that involves 5α,6α-epoxycholest-7-en-3β-ol, a primary free radical oxidation product of 7-DHC, and 7-cholesten-3β,5α,6β-triol is proposed. In a preliminary test, a pyrimidinol antioxidant was found to effectively suppress the formation of DHCEO in SLOS fibroblasts.
Figures


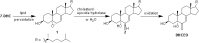

References
-
- Irons M., Elias E. R., Salen G., Tint G. S., Batta A. K. 1993. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 341: 1414. - PubMed
-
- Tint G. S., Irons M., Elias E. R., Batta A. K., Frieden R., Chen T. S., Salen G. 1994. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Engl. J. Med. 330: 107–113. - PubMed
-
- Fitzky B. U., Moebius F. F., Asaoka H., Waage-Baudet H., Xu L., Xu G., Maeda N., Kluckman K., Hiller S., Yu H., et al. 2001. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J. Clin. Invest. 108: 905–915. - PMC - PubMed
-
- Krakowiak P. A., Nwokoro N. A., Wassif C. A., Battaile K. P., Nowaczyk M. J., Connor W. E., Maslen C., Steiner R. D., Porter F. D. 2000. Mutation analysis and description of sixteen RSH/Smith-Lemli-Opitz syndrome patients: polymerase chain reaction-based assays to simplify genotyping. Am. J. Med. Genet. 94: 214–227. - PubMed
-
- Waterham H. R. 2002. Inherited disorders of cholesterol biosynthesis. Clin. Genet. 61: 393–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

