Industry involvement and baseline assumptions of cost-effectiveness analyses: diagnostic accuracy of the Papanicolaou test
- PMID: 21402681
- PMCID: PMC3071415
- DOI: 10.1503/cmaj.101506
Industry involvement and baseline assumptions of cost-effectiveness analyses: diagnostic accuracy of the Papanicolaou test
Abstract
Background: Industry involvement has been associated with more favourable cost-effectiveness ratios in cost-effectiveness analyses, but the mechanisms for this association are unclear. We evaluated whether the assumed accuracy of the Papanicolaou (Pap) test was correlated with the features of cost-effectiveness analysis studies.
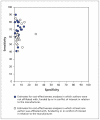
Methods: We searched PubMed (last updated April 2010) for cost-effectiveness analysis studies in which at least one strategy involved the Pap test for cervical cancer. We assessed the baseline assumed diagnostic sensitivity and specificity of the Pap test in each study and the association of these values with three levels of manufacturer involvement in the study.
Results: Among 88 analyzed cost-effectiveness analysis studies, the assumed sensitivity of the Pap test was lower in studies with manufacturer-affiliated authors, manufacturer funding or manufacturer-related competing interests versus studies without (mean sensitivity 60% v. 70%, p < 0.001). The assumed specificity of the Pap test was lower in cost-effectiveness analyses involving new screening tests (mean 93% v. 96%, p = 0.016). The assumed specificity did not differ between trials with manufacturer involvement versus those without (mean 95% v. 95%, p = 0.755).
Interpretation: The results of cost-effectiveness analyses may be affected by a downgrading of the assumed diagnostic accuracy of the standard Pap test against which newer tests or interventions are compared. New technology then seems to have more favourable results against a straw-man comparator.
Figures
Comment in
-
A glimpse into the black box of cost-effectiveness analyses.CMAJ. 2011 Apr 5;183(6):E307-8. doi: 10.1503/cmaj.110384. Epub 2011 Mar 14. CMAJ. 2011. PMID: 21402688 Free PMC article. No abstract available.
References
-
- Kassirer JP, Angell M. The journal’s policy on cost-effectiveness analyses. N Engl J Med 1994;331:669–70 - PubMed
-
- National Cancer Institute Pap test Bethesda (MD): The Institute; 2010. Available: www.cancer.gov/cancertopics/factsheet/Detection/Pap-test (accessed July 23, 2010)
-
- Arbyn M, Bergeron C, Klinkhamer P, et al. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol 2008;111:167–77 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous