Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/orai channels normally prevented by mitochondria
- PMID: 21402693
- PMCID: PMC3091227
- DOI: 10.1074/jbc.M110.198952
Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/orai channels normally prevented by mitochondria
Abstract
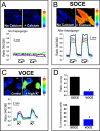
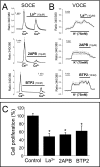
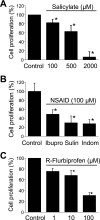
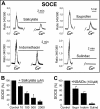
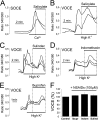
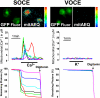
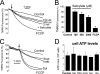
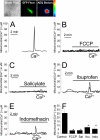
Abnormal vascular smooth muscle cell (VSMC) proliferation contributes to occlusive and proliferative disorders of the vessel wall. Salicylate and other nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit VSMC proliferation by an unknown mechanism unrelated to anti-inflammatory activity. In search for this mechanism, we have studied the effects of salicylate and other NSAIDs on subcellular Ca(2+) homeostasis and Ca(2+)-dependent cell proliferation in rat aortic A10 cells, a model of neointimal VSMCs. We found that A10 cells displayed both store-operated Ca(2+) entry (SOCE) and voltage-operated Ca(2+) entry (VOCE), the former being more important quantitatively than the latter. Inhibition of SOCE by specific Ca(2+) released-activated Ca(2+) (CRAC/Orai) channels antagonists prevented A10 cell proliferation. Salicylate and other NSAIDs, including ibuprofen, indomethacin, and sulindac, inhibited SOCE and thereby Ca(2+)-dependent, A10 cell proliferation. SOCE, but not VOCE, induced mitochondrial Ca(2+) uptake in A10 cells, and mitochondrial depolarization prevented SOCE, thus suggesting that mitochondrial Ca(2+) uptake controls SOCE (but not VOCE) in A10 cells. NSAIDs depolarized mitochondria and prevented mitochondrial Ca(2+) uptake, suggesting that they favor the Ca(2+)-dependent inactivation of CRAC/Orai channels. NSAIDs also inhibited SOCE in rat basophilic leukemia cells where mitochondrial control of CRAC/Orai is well established. NSAIDs accelerate slow inactivation of CRAC currents in rat basophilic leukemia cells under weak Ca(2+) buffering conditions but not in strong Ca(2+) buffer, thus excluding that NSAIDs inhibit SOCE directly. Taken together, our results indicate that NSAIDs inhibit VSMC proliferation by facilitating the Ca(2+)-dependent inactivation of CRAC/Orai channels which normally is prevented by mitochondria clearing of entering Ca(2+).
Figures


Similar articles
-
Mitochondrial control of store-operated Ca2+ channels in cancer: Pharmacological implications.Pharmacol Res. 2018 Sep;135:136-143. doi: 10.1016/j.phrs.2018.08.001. Epub 2018 Aug 3. Pharmacol Res. 2018. PMID: 30081178 Review.
-
TRPC1 and ORAI1 channels in colon cancer.Cell Calcium. 2019 Jul;81:59-66. doi: 10.1016/j.ceca.2019.06.003. Epub 2019 Jun 7. Cell Calcium. 2019. PMID: 31203007 Review.
-
Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes.Am J Physiol Cell Physiol. 2009 Nov;297(5):C1103-12. doi: 10.1152/ajpcell.00283.2009. Epub 2009 Aug 12. Am J Physiol Cell Physiol. 2009. PMID: 19675303 Free PMC article.
-
Calcium remodeling in colorectal cancer.Biochim Biophys Acta Mol Cell Res. 2017 Jun;1864(6):843-849. doi: 10.1016/j.bbamcr.2017.01.005. Epub 2017 Jan 10. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 28087343 Review.
-
Cell proliferation depends on mitochondrial Ca2+ uptake: inhibition by salicylate.J Physiol. 2006 Feb 15;571(Pt 1):57-73. doi: 10.1113/jphysiol.2005.100586. Epub 2005 Dec 8. J Physiol. 2006. PMID: 16339178 Free PMC article.
Cited by
-
Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation.EMBO J. 2011 Aug 16;30(19):3895-912. doi: 10.1038/emboj.2011.289. EMBO J. 2011. PMID: 21847095 Free PMC article.
-
Ryanodine receptor 2 contributes to hemorrhagic shock-induced bi-phasic vascular reactivity in rats.Acta Pharmacol Sin. 2014 Nov;35(11):1375-84. doi: 10.1038/aps.2014.83. Epub 2014 Sep 29. Acta Pharmacol Sin. 2014. PMID: 25263335 Free PMC article.
-
Mitochondria sustain store-operated currents in colon cancer cells but not in normal colonic cells: reversal by non-steroidal anti-inflammatory drugs.Oncotarget. 2017 Jul 21;8(33):55332-55352. doi: 10.18632/oncotarget.19430. eCollection 2017 Aug 15. Oncotarget. 2017. PMID: 28903423 Free PMC article.
-
Indomethacin impairs mitochondrial dynamics by activating the PKCζ-p38-DRP1 pathway and inducing apoptosis in gastric cancer and normal mucosal cells.J Biol Chem. 2019 May 17;294(20):8238-8258. doi: 10.1074/jbc.RA118.004415. Epub 2019 Apr 2. J Biol Chem. 2019. PMID: 30940726 Free PMC article.
-
Orai1 calcium channels in the vasculature.Pflugers Arch. 2012 Apr;463(5):635-47. doi: 10.1007/s00424-012-1090-2. Epub 2012 Mar 9. Pflugers Arch. 2012. PMID: 22402985 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

