ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts
- PMID: 21402694
- PMCID: PMC3089559
- DOI: 10.1074/jbc.M111.231571
ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts
Abstract
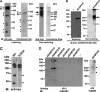
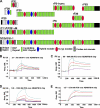
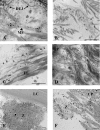
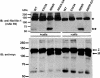
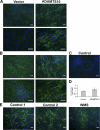
Autosomal recessive and autosomal dominant forms of Weill-Marchesani syndrome, an inherited connective tissue disorder, are caused by mutations in ADAMTS10 (encoding a secreted metalloprotease) and FBN1 (encoding fibrillin-1, which forms tissue microfibrils), respectively, yet they are clinically indistinguishable. This genetic connection prompted investigation of a potential functional relationship between ADAMTS10 and fibrillin-1. Specifically, fibrillin-1 was investigated as a potential ADAMTS10 binding partner and substrate, and the role of ADAMTS10 in influencing microfibril biogenesis was addressed. Using ligand affinity blotting and surface plasmon resonance, recombinant ADAMTS10 was found to bind to fibrillin-1 with a high degree of specificity and with high affinity. Two sites of ADAMTS10 binding to fibrillin-1 were identified, one toward the N terminus and another in the C-terminal half of fibrillin-1. Confocal microscopy and immunoelectron microscopy localized ADAMTS10 to fibrillin-1-containing microfibrils in human tissues. Furin-activated ADAMTS10 could cleave fibrillin-1, but innate resistance of ADAMTS10 zymogen to propeptide excision by furin was observed, suggesting that, unless activated, ADAMTS10 is an inefficient fibrillinase. To investigate the role of ADAMTS10 in microfibril biogenesis, fetal bovine nuchal ligament cells were cultured in the presence or absence of ADAMTS10. Exogenously added ADAMTS10 led to accelerated fibrillin-1 microfibril biogenesis. Conversely, fibroblasts obtained from a Weill-Marchesani syndrome patient with ADAMTS10 mutations deposited fibrillin-1 microfibrils sparsely compared with unaffected control cells. Taken together, these findings suggest that ADAMTS10 participates in microfibril biogenesis rather than in fibrillin-1 turnover.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

