Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia
- PMID: 21402714
- PMCID: PMC3108904
- DOI: 10.1158/1078-0432.CCR-10-2879
Phase II study of dasatinib in relapsed or refractory chronic lymphocytic leukemia
Abstract
Purpose: Chronic lymphocytic leukemia (CLL) cells treated with dasatinib in vitro undergo apoptosis via inhibition of Lyn kinase. Thus, in this study we tested the activity of dasatinib in patients with relapsed CLL.
Experimental design: Patients were eligible for this phase II trial if they had documented CLL/SLL and had failed at least 1 prior therapy with a fludarabine-containing regimen and if they required therapy according to NCI-WG criteria. The starting dose of dasatinib was 140 mg daily.
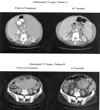
Results: Fifteen patients were enrolled, with a median age of 59 and a median of 3 prior regimens. All patients had received fludarabine, and 5 were fludarabine-refractory. Eleven of the 15 (73%) had high risk del(11q) or del(17p) cytogenetics. The primary toxicity was myelosuppression, with grade 3 or 4 neutropenia and thrombocytopenia in 10 and 6 patients, respectively. Partial responses by NCI-WG criteria were achieved in 3 of the 15 patients (20%; 90% CI: 6-44). Among the remaining 12 patients, 5 had nodal responses by physical exam, and 1 patient had a nodal and lymphocyte response but with severe myelosuppression. Pharmacodynamic studies indicated apoptosis in peripheral blood CLL cells within 3 to 6 hours after dasatinib administration, associated with downregulation of Syk (spleen tyrosine kinase) mRNA.
Conclusions: Dasatinib as a single agent has activity in relapsed and refractory CLL.
©2011 AACR.
Conflict of interest statement
Conflict-of-interest disclosure: This study is an investigator-initiated study supported by per patient funding from Bristol Myers Squibb, which also supplied dasatinib. P.C.A. received $1,500 for attending a Bristol-Myers Squibb Advisory Board Meeting regarding Hematologic Malignancies in 2007. J.R.B. served as a consultant for Calistoga, Celgene, and Genentech and received research funding from Celgene and Genzyme. The other authors have no conflict-of-interest to report.
Figures

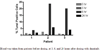

References
-
- Zweibel J, Ho P, Sepelak SB, Saunders J, Cheson BD, Nelson A. Clinical trials referral resource. Clinical trials with perillyl alcohol. Oncology (Williston Park) 1997;11(12):1817–1820. - PubMed
-
- Wierda W, O'Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4070–4078. - PubMed
-
- Keating MJ, O'Brien S, Kontoyiannis D, Plunkett W, Koller C, Beran M, et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43(9):1755–1762. - PubMed
-
- Perkins JG, Flynn JM, Howard RS, Byrd JC. Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: implications for clinical trials in this patient population. Cancer. 2002;94(7):2033–2039. - PubMed
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–815. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

