Mechanisms of necroptosis in T cells
- PMID: 21402742
- PMCID: PMC3135356
- DOI: 10.1084/jem.20110251
Mechanisms of necroptosis in T cells
Abstract
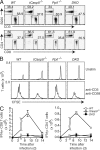
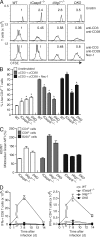
Cell populations are regulated in size by at least two forms of apoptosis. More recently, necroptosis, a parallel, nonapoptotic pathway of cell death, has been described, and this pathway is invoked in the absence of caspase 8. In caspase 8-deficient T cells, necroptosis occurs as the result of antigen receptor-mediated activation. Here, through a genetic analysis, we show that necroptosis in caspase 8-deficient T cells is related neither to the programmed necrosis as defined by the requirement for mitochondrial cyclophilin D nor to autophagy as defined by the requirement for autophagy-related protein 7. Rather, survival of caspase 8-defective T cells can be completely rescued by loss of receptor-interacting serine-threonine kinase (Ripk) 3. Additionally, complementation of a T cell-specific caspase 8 deficiency with a loss of Ripk3 gives rise to lymphoproliferative disease reminiscent of lpr or gld mice. In conjunction with previous work, we conclude that necroptosis in antigen-stimulated caspase 8-deficient T cells is the result of a novel Ripk1- and Ripk3-mediated pathway of cell death.
Figures
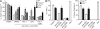

Comment in
-
Cell death and immunity: caspase 8 and RIPK3 play with life and death.Nat Rev Immunol. 2011 May;11(5):300-1. doi: 10.1038/nri2973. Epub 2011 Apr 1. Nat Rev Immunol. 2011. PMID: 21494266 No abstract available.
References
-
- Arechiga A.F., Bell B.D., Solomon J.C., Chu I.H., Dubois C.L., Hall B.E., George T.C., Coder D.M., Walsh C.M. 2005. Cutting edge: FADD is not required for antigen receptor-mediated NF-kappaB activation. J. Immunol. 175:7800–7804 - PubMed
-
- Arechiga A.F., Bell B.D., Leverrier S., Weist B.M., Porter M., Wu Z., Kanno Y., Ramos S.J., Ong S.T., Siegel R., Walsh C.M. 2007. A Fas-associated death domain protein/caspase-8-signaling axis promotes S-phase entry and maintains S6 kinase activity in T cells responding to IL-2. J. Immunol. 179:5291–5300 - PubMed
-
- Baines C.P., Kaiser R.A., Purcell N.H., Blair N.S., Osinska H., Hambleton M.A., Brunskill E.W., Sayen M.R., Gottlieb R.A., Dorn G.W., et al. 2005. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 434:658–662 10.1038/nature03434 - DOI - PubMed
-
- Beisner D.R., Ch’en I.L., Kolla R.V., Hoffmann A., Hedrick S.M. 2005. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J. Immunol. 175:3469–3473 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

