Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence
- PMID: 21402762
- PMCID: PMC3125851
- DOI: 10.1128/IAI.00117-11
Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence
Abstract
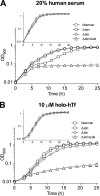
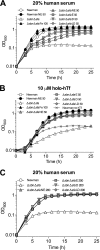
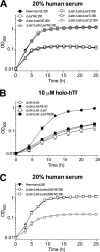
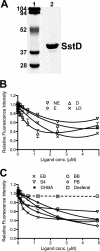
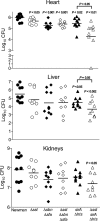
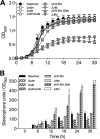
Staphylococcus aureus is a frequent cause of bloodstream, respiratory tract, and skin and soft tissue infections. In the bloodstream, the iron-binding glycoprotein transferrin circulates to provide iron to cells throughout the body, but its iron-binding properties make it an important component of innate immunity. It is well established that siderophores, with their high affinity for iron, in many instances can remove iron from transferrin as a means to promote proliferation of bacterial pathogens. It is also established that catecholamine hormones can interfere with the iron-binding properties of transferrin, thus allowing infectious bacteria access to this iron pool. The present study demonstrates that S. aureus can use either of two carboxylate-type siderophores, staphyloferrin A and staphyloferrin B, via the transporters Hts and Sir, respectively, to access the transferrin iron pool. Growth of staphyloferrin-producing S. aureus in serum or in the presence of holotransferrin was not enhanced in the presence of catecholamines. However, catecholamines significantly enhanced the growth of staphyloferrin-deficient S. aureus in human serum or in the presence of human holotransferrin. It was further demonstrated that the Sst transporter was essential for this activity as well as for the utilization of bacterial catechol siderophores. The substrate binding protein SstD was shown to interact with ferrated catecholamines and catechol siderophores, with low to submicromolar affinities. Experiments involving mice challenged intravenously with wild-type S. aureus and isogenic mutants demonstrated that the combination of Hts, Sir, and Sst transport systems was required for full virulence of S. aureus.
Figures

References
-
- Aisen P., Leibman A., Zweier J. 1978. Stoichiometric and site characteristics of the binding of iron to human transferrin. J. Biol. Chem. 253:1930–1937 - PubMed
-
- Beasley F. C., et al. 2009. Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol. Microbiol. 72:947–963 - PubMed
-
- Brickman T. J., Armstrong S. K. 2010. Iron uptake systems in pathogenic Bordetella, p. 65–86 In Cornelis P., Andrews S. C. (ed.), Iron uptake and homeostasis in microorganisms. Caister Academic Press, Norfolk, United Kingdom
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

