Essential role for neutrophils in pathogenesis and adaptive immunity in Chlamydia caviae ocular infections
- PMID: 21402767
- PMCID: PMC3088137
- DOI: 10.1128/IAI.01257-10
Essential role for neutrophils in pathogenesis and adaptive immunity in Chlamydia caviae ocular infections
Abstract
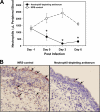
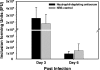
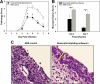
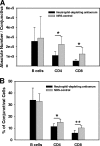
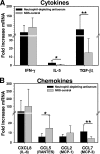
Trachoma, the world's leading cause of preventable blindness, is produced by chronic ocular infection with Chlamydia trachomatis, an obligate intracellular bacterium. While many studies have focused on immune mechanisms for trachoma during chronic stages of infection, less research has targeted immune mechanisms in primary ocular infections, events that could impact chronic responses. The goal of this study was to investigate the function of neutrophils during primary chlamydial ocular infection by using the guinea pig model of Chlamydia caviae inclusion conjunctivitis. We hypothesized that neutrophils help modulate the adaptive response and promote host tissue damage. To test these hypotheses, guinea pigs with primary C. caviae ocular infections were depleted of neutrophils by using rabbit antineutrophil antiserum, and immune responses and immunopathology were evaluated during the first 7 days of infection. Results showed that neutrophil depletion dramatically decreased ocular pathology, both clinically and histologically. The adaptive response was also altered, with increased C. caviae-specific IgA titers in tears and serum and decreased numbers of CD4(+) and CD8(+) T cells in infected conjunctivae. Additionally, there were changes in conjunctival chemokines and cytokines, such as increased expression of IgA-promoting interleukin-5 and anti-inflammatory transforming growth factor β, along with decreased expression of T cell-recruiting CCL5 (RANTES). This study, the first to investigate the role of neutrophils in primary chlamydial ocular infection, indicates a previously unappreciated role for neutrophils in modulating the adaptive response and suggests a prominent role for neutrophils in chlamydia-associated ocular pathology.
Figures
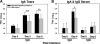
References
-
- Abbas A. K., Lichtman A. H., Pillai S. 2007. Cellular and molecular immunology. Saunders Elsevier, Philadelphia, PA
-
- Ahmad A., Dawson C. R., Yoneda C., Togni B., Schachter J. 1977. Resistance to reinfection with a chlamydial agent (guinea pig inclusion conjunctivitis agent). Invest. Ophthalmol. Vis. Sci. 16:549–553 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

