Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane
- PMID: 21402783
- PMCID: PMC3133353
- DOI: 10.1128/MCB.05051-11
Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane
Abstract
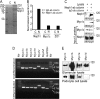
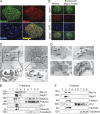
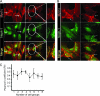
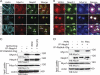
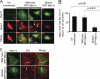
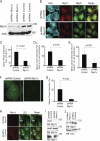
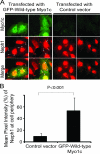
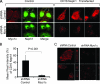
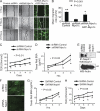
The podocyte proteins Neph1 and nephrin organize a signaling complex at the podocyte cell membrane that forms the structural framework for a functional glomerular filtration barrier. Mechanisms regulating the movement of these proteins to and from the membrane are currently unknown. This study identifies a novel interaction between Neph1 and the motor protein Myo1c, where Myo1c plays an active role in targeting Neph1 to the podocyte cell membrane. Using in vivo and in vitro experiments, we provide data supporting a direct interaction between Neph1 and Myo1c which is dynamic and actin dependent. Unlike wild-type Myo1c, the membrane localization of Neph1 was significantly reduced in podocytes expressing dominant negative Myo1c. In addition, Neph1 failed to localize at the podocyte cell membrane and cell junctions in Myo1c-depleted podocytes. We further demonstrate that similarly to Neph1, Myo1c also binds nephrin and reduces its localization at the podocyte cell membrane. A functional analysis of Myo1c knockdown cells showed defects in cell migration, as determined by a wound assay. In addition, the ability to form tight junctions was impaired in Myo1c knockdown cells, as determined by transepithelial electric resistance (TER) and bovine serum albumin (BSA) permeability assays. These results identify a novel Myo1c-dependent molecular mechanism that mediates the dynamic organization of Neph1 and nephrin at the slit diaphragm and is critical for podocyte function.
Figures
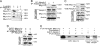

References
-
- Bahler M., Rhoads A. 2002. Calmodulin signaling via the IQ motif. FEBS Lett. 513:107–113 - PubMed
-
- Barletta G. M., Kovari I. A., Verma R. K., Kerjaschki D., Holzman L. B. 2003. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J. Biol. Chem. 278:19266–19271 - PubMed
-
- Barylko B., Jung G., Albanesi J. P. 2005. Structure, function, and regulation of myosin 1C. Acta Biochim. Pol. 52:373–380 - PubMed
-
- Benzing T. 2004. Signaling at the slit diaphragm. J. Am. Soc. Nephrol. 15:1382–1391 - PubMed
-
- Bose A., et al. 2002. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420:821–824 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
