Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy
- PMID: 21402784
- PMCID: PMC3133349
- DOI: 10.1128/MCB.05234-11
Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy
Abstract
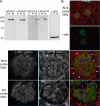
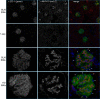
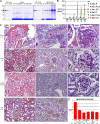
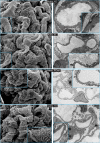
Genome-wide association studies linked single-nucleotide polymorphisms (SNPs) at the MYH9 locus to chronic kidney disease among African-Americans, particularly glomerular diseases such as HIV nephropathy and idiopathic focal and segmental glomerulosclerosis (FSGS). However, these MYH9 SNPs are intronic, and despite extensive sequencing, a causal variant remains elusive. To investigate the role of MYH9 in kidney disease, we selectively deleted Myh9 from mouse podocytes and found that mutant C57BL/6 mice did not develop renal insufficiency or proteinuria compared to control littermates, even when the mice were aged for 9 months. To explain the surprisingly normal phenotype, we considered genetic redundancy with the paralog Myh10 in podocytes, but we found that Myh10 was not expressed in podocytes in Myh9-deficient or control mice. We tested whether Myh9 podocyte deletion predisposed mice to glomerulopathy in response to injury by doxorubicin hydrochloride (Adriamycin), and we found that Myh9 podocyte-deleted mice developed proteinuria and glomerulosclerosis, while control mice were resistant. In summary, Myh9 podocyte deletion in C57BL/6 mice results in susceptibility to experimental doxorubicin hydrochloride glomerulopathy. We review evidence that MYH9 dysfunction in humans results in similar susceptibility and place our data, the first examination of Myh9 kidney disease in experimental animals, in the context of recent findings in human kidney disease, including the role of APOL1.
Figures

References
-
- Arrondel C., et al. 2002. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J. Am. Soc. Nephrol. 13:65–74 - PubMed
-
- Bao J., Jana S. S., Adelstein R. S. 2005. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J. Biol. Chem. 280:19594–19599 - PubMed
-
- Conti M. A., Adelstein R. S. 2008. Nonmuscle myosin II moves in new directions. J. Cell Sci. 121:11–18 - PubMed
-
- Conti M. A., Even-Ram S., Liu C., Yamada K. M., Adelstein R. S. 2004. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 279:41263–41266 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
