Microbiota regulates immune defense against respiratory tract influenza A virus infection
- PMID: 21402903
- PMCID: PMC3069176
- DOI: 10.1073/pnas.1019378108
Microbiota regulates immune defense against respiratory tract influenza A virus infection
Abstract
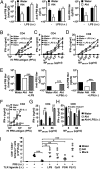
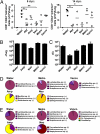
Although commensal bacteria are crucial in maintaining immune homeostasis of the intestine, the role of commensal bacteria in immune responses at other mucosal surfaces remains less clear. Here, we show that commensal microbiota composition critically regulates the generation of virus-specific CD4 and CD8 T cells and antibody responses following respiratory influenza virus infection. By using various antibiotic treatments, we found that neomycin-sensitive bacteria are associated with the induction of productive immune responses in the lung. Local or distal injection of Toll-like receptor (TLR) ligands could rescue the immune impairment in the antibiotic-treated mice. Intact microbiota provided signals leading to the expression of mRNA for pro-IL-1β and pro-IL-18 at steady state. Following influenza virus infection, inflammasome activation led to migration of dendritic cells (DCs) from the lung to the draining lymph node and T-cell priming. Our results reveal the importance of commensal microbiota in regulating immunity in the respiratory mucosa through the proper activation of inflammasomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
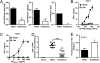

Comment in
-
Antiviral immunity: flora against the flu.Nat Rev Immunol. 2011 May;11(5):304-5. doi: 10.1038/nri2982. Nat Rev Immunol. 2011. PMID: 21508980 No abstract available.
References
-
- Fitzgerald KA. NLR-containing inflammasomes: Central mediators of host defense and inflammation. Eur J Immunol. 2010;40:595–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

