Two ubiquitin ligases, APC/C-Cdh1 and SKP1-CUL1-F (SCF)-beta-TrCP, sequentially regulate glycolysis during the cell cycle
- PMID: 21402913
- PMCID: PMC3069186
- DOI: 10.1073/pnas.1102247108
Two ubiquitin ligases, APC/C-Cdh1 and SKP1-CUL1-F (SCF)-beta-TrCP, sequentially regulate glycolysis during the cell cycle
Abstract
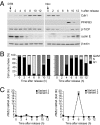
During cell proliferation, the abundance of the glycolysis-promoting enzyme, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase, isoform 3 (PFKFB3), is controlled by the ubiquitin ligase APC/C-Cdh1 via a KEN box. We now demonstrate in synchronized HeLa cells that PFKFB3, which appears in mid-to-late G1, is essential for cell division because its silencing prevents progression into S phase. In cells arrested by glucose deprivation, progression into S phase after replacement of glucose occurs only when PFKFB3 is present or is substituted by the downstream glycolytic enzyme 6-phosphofructo-1-kinase. PFKFB3 ceases to be detectable during late G1/S despite the absence of Cdh1; this disappearance is prevented by proteasomal inhibition. PFKFB3 contains a DSG box and is therefore a potential substrate for SCF-β-TrCP, a ubiquitin ligase active during S phase. In synchronized HeLa cells transfected with PFKFB3 mutated in the KEN box, the DSG box, or both, we established the breakdown routes of the enzyme at different stages of the cell cycle and the point at which glycolysis is enhanced. Thus, the presence of PFKFB3 is tightly controlled to ensure the up-regulation of glycolysis at a specific point in G1. We suggest that this up-regulation of glycolysis and its associated events represent the nutrient-sensitive restriction point in mammalian cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells.Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):21069-74. doi: 10.1073/pnas.1117500108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106309 Free PMC article.
-
The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1.Nat Cell Biol. 2009 Jun;11(6):747-52. doi: 10.1038/ncb1881. Epub 2009 May 17. Nat Cell Biol. 2009. PMID: 19448625
-
Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis and glutaminolysis with transition to S phase in human T lymphocytes.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18868-73. doi: 10.1073/pnas.1012362107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921411 Free PMC article.
-
Glycolysis: a bioenergetic or a survival pathway?Trends Biochem Sci. 2010 Mar;35(3):145-9. doi: 10.1016/j.tibs.2009.10.006. Epub 2009 Dec 16. Trends Biochem Sci. 2010. PMID: 20006513 Review.
-
Fulfilling the metabolic requirements for cell proliferation.Biochem J. 2012 Aug 15;446(1):1-7. doi: 10.1042/BJ20120427. Biochem J. 2012. PMID: 22835215 Review.
Cited by
-
AMP-activated protein kinase is dispensable for maintaining ATP levels and for survival following inhibition of glycolysis, but promotes tumour engraftment of Ras-transformed fibroblasts.Oncotarget. 2015 May 20;6(14):11833-47. doi: 10.18632/oncotarget.3738. Oncotarget. 2015. PMID: 26059436 Free PMC article.
-
E3 Ligase SCFβTrCP-induced DYRK1A Protein Degradation Is Essential for Cell Cycle Progression in HEK293 Cells.J Biol Chem. 2016 Dec 16;291(51):26399-26409. doi: 10.1074/jbc.M116.717553. Epub 2016 Nov 2. J Biol Chem. 2016. PMID: 27807027 Free PMC article.
-
Glucose to lactate shift reprograms CDK-dependent mitotic decisions and its communication with MAPK Sty1 in Schizosaccharomyces pombe.Biol Open. 2023 Oct 15;12(10):bio060145. doi: 10.1242/bio.060145. Epub 2023 Oct 24. Biol Open. 2023. PMID: 37787465 Free PMC article.
-
Cone photoreceptor phosphodiesterase PDE6H inhibition regulates cancer cell growth and metabolism, replicating the dark retina response.Cancer Metab. 2024 Feb 13;12(1):5. doi: 10.1186/s40170-023-00326-y. Cancer Metab. 2024. PMID: 38350962 Free PMC article.
-
Colon cancer associated genes exhibit signatures of positive selection at functionally significant positions.BMC Evol Biol. 2012 Jul 12;12:114. doi: 10.1186/1471-2148-12-114. BMC Evol Biol. 2012. PMID: 22788692 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

