Physical effects underlying the transition from primitive to modern cell membranes
- PMID: 21402937
- PMCID: PMC3069173
- DOI: 10.1073/pnas.1100498108
Physical effects underlying the transition from primitive to modern cell membranes
Abstract
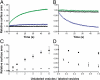
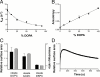
To understand the emergence of Darwinian evolution, it is necessary to identify physical mechanisms that enabled primitive cells to compete with one another. Whereas all modern cell membranes are composed primarily of diacyl or dialkyl glycerol phospholipids, the first cell membranes are thought to have self-assembled from simple, single-chain lipids synthesized in the environment. We asked what selective advantage could have driven the transition from primitive to modern membranes, especially during early stages characterized by low levels of membrane phospholipid. Here we demonstrate that surprisingly low levels of phospholipids can drive protocell membrane growth during competition for single-chain lipids. Growth results from the decreasing fatty acid efflux from membranes with increasing phospholipid content. The ability to synthesize phospholipids from single-chain substrates would have therefore been highly advantageous for early cells competing for a limited supply of lipids. We show that the resulting increase in membrane phospholipid content would have led to a cascade of new selective pressures for the evolution of metabolic and transport machinery to overcome the reduced membrane permeability of diacyl lipid membranes. The evolution of phospholipid membranes could thus have been a deterministic outcome of intrinsic physical processes and a key driving force for early cellular evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

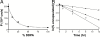
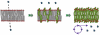
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

