Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior
- PMID: 21402951
- PMCID: PMC3078408
- DOI: 10.1073/pnas.1015551108
Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior
Abstract
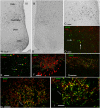
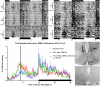
Food anticipatory behavior (FAA) is induced by limiting access to food for a few hours daily. Animals anticipate this scheduled meal event even without the suprachiasmatic nucleus (SCN), the biological clock. Consequently, a food-entrained oscillator has been proposed to be responsible for meal time estimation. Recent studies suggested the dorsomedial hypothalamus (DMH) as the site for this food-entrained oscillator, which has led to considerable controversy in the literature. Herein we demonstrate by means of c-Fos immunohistochemistry that the neuronal activity of the suprachiasmatic nucleus (SCN), which signals the rest phase in nocturnal animals, is reduced when animals anticipate the scheduled food and, simultaneously, neuronal activity within the DMH increases. Using retrograde tracing and confocal analysis, we show that inhibition of SCN neuronal activity is the consequence of activation of GABA-containing neurons in the DMH that project to the SCN. Next, we show that DMH lesions result in a loss or diminution of FAA, simultaneous with increased activity in the SCN. A subsequent lesion of the SCN restored FAA. We conclude that in intact animals, FAA may only occur when the DMH inhibits the activity of the SCN, thus permitting locomotor activity. As a result, FAA originates from a neuronal network comprising an interaction between the DMH and SCN. Moreover, this study shows that the DMH-SCN interaction may serve as an intrahypothalamic system to gate activity instead of rest overriding circadian predetermined temporal patterns.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schwartz WJ, Reppert SM, Eagan SM, Moore-Ede MC. In vivo metabolic activity of the suprachiasmatic nuclei: A comparative study. Brain Res. 1983;274:184–187. - PubMed
-
- Houben T, Deboer T, van Oosterhout F, Meijer JH. Correlation with behavioral activity and rest implies circadian regulation by SCN neuronal activity levels. J Biol Rhythms. 2009;24:477–487. - PubMed
-
- Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. 1979;25:545–554. - PubMed
-
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

