Progenitor cell-based treatment of the pediatric myelin disorders
- PMID: 21403006
- PMCID: PMC3358919
- DOI: 10.1001/archneurol.2011.46
Progenitor cell-based treatment of the pediatric myelin disorders
Abstract
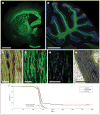
The childhood leukodystrophies are characterized by neonatal or childhood deficiencies in myelin production or maintenance; these may be due to hereditary defects in genes for myelin maintenance, as in Pelizaeus-Merzbacher disease, or to enzymatic deficiencies resulting in substrate misaccumulation or misprocessing, as in the lysosomal storage disorders. Regardless of their respective etiologies, these disorders are essentially all manifested by a profound deterioration in neurological function with age. A congenital deficit in forebrain myelination is also noted in children with the periventricular leukomalacia of cerebral palsy, which yields a more static morbidity. In light of the wide range of disorders to which congenital hypomyelination or postnatal demyelination may contribute, and the relative homogeneity of oligodendrocytes and their progenitors, the leukodystrophies may be especially attractive targets for cell-based therapeutic strategies. As a result, glial progenitor cells, which can give rise to new myelinogenic oligodendrocytes, have become of great interest as potential vectors for the restoration of myelin to the dysmyelinated brain and spinal cord. In addition, by distributing throughout the neuraxis after perinatal graft, and giving rise to astrocytes as well as oligodendrocytes, glial progenitor cells may be of great utility in rectifying the dysmyelination-associated enzymatic deficiencies of the lysosomal storage disorders.
Figures
References
-
- Goldman SA. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23(7):862–871. - PubMed
-
- Koch P, Kokaia Z, Lindvall O, Brüstle O. Emerging concepts in neural stem cell research: autologous repair and cell-based disease modelling. Lancet Neurol. 2009;8(9):819–829. - PubMed
-
- Martino G, Franklin RJ, Van Evercooren AB, Kerr DA Stem Cells in Multiple Sclerosis (STEMS) Consensus Group. Stem cell transplantation in multiple sclerosis: current status and future prospects. Nat Rev Neurol. 2010;6(5):247–255. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

