Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations
- PMID: 21403054
- PMCID: PMC3733115
- DOI: 10.7326/0003-4819-154-9-201105030-00336
Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations
Erratum in
-
Correction: Comparative effectiveness and safety of medications for type 2 diabetes.Ann Intern Med. 2011 Jul 5;155(1):67-8. doi: 10.7326/0003-4819-155-1-201107050-00016. Ann Intern Med. 2011. PMID: 21858938 No abstract available.
Abstract
Background: Given the increase in medications for type 2 diabetes mellitus, clinicians and patients need information about their effectiveness and safety to make informed choices.
Purpose: To summarize the benefits and harms of metformin, second-generation sulfonylureas, thiazolidinediones, meglitinides, dipeptidyl peptidase-4 (DPP-4) inhibitors, and glucagon-like peptide-1 receptor agonists, as monotherapy and in combination, to treat adults with type 2 diabetes.
Data sources: MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials were searched from inception through April 2010 for English-language observational studies and trials. The MEDLINE search was updated to December 2010 for long-term clinical outcomes.
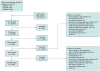
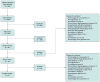
Study selection: Two reviewers independently screened reports and identified 140 trials and 26 observational studies of head-to-head comparisons of monotherapy or combination therapy that reported intermediate or long-term clinical outcomes or harms.
Data extraction: Two reviewers following standardized protocols serially extracted data, assessed applicability, and independently evaluated study quality.
Data synthesis: Evidence on long-term clinical outcomes (all-cause mortality, cardiovascular disease, nephropathy, and neuropathy) was of low strength or insufficient. Most medications decreased the hemoglobin A(1c) level by about 1 percentage point and most 2-drug combinations produced similar reductions. Metformin was more efficacious than the DPP-4 inhibitors, and compared with thiazolidinediones or sulfonylureas, the mean differences in body weight were about -2.5 kg. Metformin decreased low-density lipoprotein cholesterol levels compared with pioglitazone, sulfonylureas, and DPP-4 inhibitors. Sulfonylureas had a 4-fold higher risk for mild or moderate hypoglycemia than metformin alone and, in combination with metformin, had more than a 5-fold increased risk compared with metformin plus thiazolidinediones. Thiazolidinediones increased risk for congestive heart failure compared with sulfonylureas and increased risk for bone fractures compared with metformin. Diarrhea occurred more often with metformin than with thiazolidinediones.
Limitations: Only English-language publications were reviewed. Some studies may have selectively reported outcomes. Many studies were small, were of short duration, and had limited ability to assess clinically important harms and benefits.
Conclusion: Evidence supports metformin as a first-line agent to treat type 2 diabetes. Most 2-drug combinations similarly reduce hemoglobin A(1c) levels, but some increased risk for hypoglycemia and other adverse events.
Primary funding source: Agency for Healthcare Research and Quality.
Conflict of interest statement
Figures

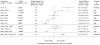
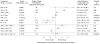
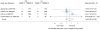


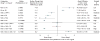

Comment in
-
ACP Journal Club. Review: oral drugs for type 2 diabetes, alone or in combination, have different relative benefits and harms for surrogate endpoints.Ann Intern Med. 2011 Aug 16;155(4):JC2-4. doi: 10.7326/0003-4819-155-4-201108160-02004. Ann Intern Med. 2011. PMID: 21844537 No abstract available.
-
Correction: Comparative effectiveness and safety of medications for type 2 diabetes.Ann Intern Med. 2011 Jul 5;155(1):67-8. doi: 10.7326/0003-4819-155-1-201107050-00016. Ann Intern Med. 2011. PMID: 21858938 No abstract available.
-
An update on the comparative effectiveness and safety of medications for type 2 diabetes.Ann Intern Med. 2011 Oct 18;155(8):562-3; author reply 563. doi: 10.7326/0003-4819-155-8-201110180-00020. Ann Intern Med. 2011. PMID: 22007052 No abstract available.
References
-
- United Kingdom Prospective Diabetes Study 24: a 6-year, randomized controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. United Kingdom Prospective Diabetes Study Group. Ann Intern Med. 1998;128:165–75. - PubMed
-
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–17. - PubMed
-
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. - PubMed
-
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
