Human cardiac stem cell differentiation is regulated by a mircrine mechanism
- PMID: 21403094
- PMCID: PMC3066266
- DOI: 10.1161/CIRCULATIONAHA.110.982918
Human cardiac stem cell differentiation is regulated by a mircrine mechanism
Erratum in
-
Correction.Circulation. 2015 Dec 1;132(22):e362. doi: 10.1161/CIR.0000000000000340. Circulation. 2015. PMID: 26621665 No abstract available.
Retraction in
-
Retraction of: Human Cardiac Stem Cell Differentiation Is Regulated by a Mircrine Mechanism.Circulation. 2019 Feb 12;139(7):e38. doi: 10.1161/CIR.0000000000000644. Circulation. 2019. PMID: 30586777 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern.Circ Res. 2019 Jan 18;124(2):e4-e5. doi: 10.1161/RES.0000000000000241. Circ Res. 2019. PMID: 30582460 No abstract available.
-
Expression of Concern.Circulation. 2019 Jan 15;139(3):e5-e6. doi: 10.1161/CIR.0000000000000639. Circulation. 2019. PMID: 30615475 No abstract available.
Abstract
Background: Cardiac stem cells (CSCs) delivered to the infarcted heart generate a large number of small fetal-neonatal cardiomyocytes that fail to acquire the differentiated phenotype. However, the interaction of CSCs with postmitotic myocytes results in the formation of cells with adult characteristics.
Methods and results: On the basis of results of in vitro and in vivo assays, we report that the commitment of human CSCs (hCSCs) to the myocyte lineage and the generation of mature working cardiomyocytes are influenced by microRNA-499 (miR-499), which is barely detectable in hCSCs but is highly expressed in postmitotic human cardiomyocytes. miR-499 traverses gap junction channels and translocates to structurally coupled hCSCs favoring their differentiation into functionally competent cells. Expression of miR-499 in hCSCs represses the miR-499 target genes Sox6 and Rod1, enhancing cardiomyogenesis in vitro and after infarction in vivo. Although cardiac repair was detected in all cell-treated infarcted hearts, the aggregate volume of the regenerated myocyte mass and myocyte cell volume were greater in animals injected with hCSCs overexpressing miR-499. Treatment with hCSCs resulted in an improvement in ventricular function, consisting of a better preservation of developed pressure and positive and negative dP/dt after infarction. An additional positive effect on cardiac performance occurred with miR-499, pointing to enhanced myocyte differentiation/hypertrophy as the mechanism by which miR-499 potentiated the restoration of myocardial mass and function in the infarcted heart.
Conclusions: The recognition that miR-499 promotes the differentiation of hCSCs into mechanically integrated cardiomyocytes has important clinical implications for the treatment of human heart failure.
Figures

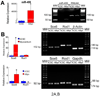



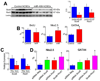


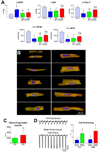
Comment in
-
Letter by Sluijter et al regarding article, "Human cardiac stem cell differentiation is regulated by a mircrine mechanism".Circulation. 2011 Oct 25;124(17):e456; author's reply e457. doi: 10.1161/CIRCULATIONAHA.111.037655. Circulation. 2011. PMID: 22025645 No abstract available.
References
-
- Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Müller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. - PubMed
-
- Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. - PubMed
-
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG017042/AG/NIA NIH HHS/United States
- R01 HL111183/HL/NHLBI NIH HHS/United States
- R01 HL075480/HL/NHLBI NIH HHS/United States
- P01 HL092868/HL/NHLBI NIH HHS/United States
- R01 AG037490/AG/NIA NIH HHS/United States
- R01 HL091021/HL/NHLBI NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R01 HL105532/HL/NHLBI NIH HHS/United States
- R01 AG037495/AG/NIA NIH HHS/United States
- R01 HL065577/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
- P01 AG023071/AG/NIA NIH HHS/United States
- R21 HL094894/HL/NHLBI NIH HHS/United States
- R01 HL065573/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

