Microglia demonstrate age-dependent interaction with amyloid-β fibrils
- PMID: 21403390
- PMCID: PMC3295838
- DOI: 10.3233/JAD-2011-101014
Microglia demonstrate age-dependent interaction with amyloid-β fibrils
Abstract
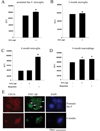
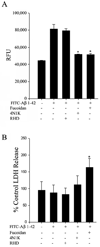
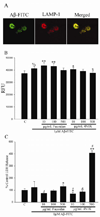
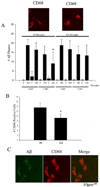
Alzheimer's disease (AD) is an age-associated disease characterized by increased accumulation of extracellular amyloid-β (Aβ) plaques within the brain. Histological examination has also revealed profound microglial activation in diseased brains often in association with these fibrillar peptide aggregates. The paradoxical presence of increased, reactive microglia yet accumulating extracellular debris suggests that these cells may be phagocytically compromised during disease. Prior work has demonstrated that primary microglia from adult mice are unable to phagocytose fibrillar Aβ1-42 in vitro when compared to microglia cultured from early postnatal animals. These data suggest that microglia undergo an age-associated decrease in microglial ability to interact with Aβ fibrils. In order to better define a temporal profile of microglia-Aβ interaction, acutely isolated, rather than cultured, microglia from 2 month, 6 month, and postnatal day 0 C57BL/6 mice were compared. Postnatal day 0 microglia demonstrated a CD47 dependent ability to phagocytose Aβ fibrils that was lost by 6 months. This corresponded with the ability of postnatal day 0 but not adult microglia to decrease Aβ immunoreactive plaque load from AD sections in vitro. In spite of limited Aβ uptake ability, adult microglia had functional phagocytic uptake of bacterial bioparticles and demonstrated the ability to adhere to both Aβ plaques and in vitro fibrillized Aβ. These data demonstrate a temporal profile of specifically Aβ-microglia interaction with a critical developmental period at 6 months in which cells remain able to interact with Aβ fibrils but lose their ability to phagocytose it.
Figures


References
-
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. - PubMed
-
- Styren SD, Civin WH, Rogers J. Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer's disease brain. Exp Neurol. 1990;110:93–104. - PubMed
-
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. - PubMed
-
- Sasaki A, Yamaguchi H, Ogawa A, Sugihara S, Nakazato Y. Microglial activation in early stages of amyloid beta protein deposition. Acta Neuropathol. 1997;94:316–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

