Good COP1 or bad COP1? In vivo veritas
- PMID: 21403396
- PMCID: PMC3069793
- DOI: 10.1172/JCI57080
Good COP1 or bad COP1? In vivo veritas
Abstract
The evolutionarily conserved protein COP1 has been shown to operate as an E3 ubiquitin ligase complex, and a number of putative substrates have been identified, including the c-JUN oncoprotein and p53 tumor suppressor protein. New work by Migliorini and colleagues described in the current issue of JCI demonstrates that COP1 acts as a tumor suppressor in vivo and does so, at least in part, by promoting the destruction of c-JUN. These findings challenge the view that COP1 regulates p53 stability and call into question the wisdom of developing COP1 inhibitors as potential anticancer agents.
Figures
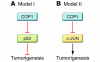
Comment on
-
Cop1 constitutively regulates c-Jun protein stability and functions as a tumor suppressor in mice.J Clin Invest. 2011 Apr;121(4):1329-43. doi: 10.1172/JCI45784. Epub 2011 Mar 14. J Clin Invest. 2011. PMID: 21403399 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

