Antibacterial peptides from plants: what they are and how they probably work
- PMID: 21403856
- PMCID: PMC3049328
- DOI: 10.1155/2011/250349
Antibacterial peptides from plants: what they are and how they probably work
Abstract
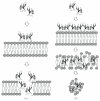
Plant antibacterial peptides have been isolated from a wide variety of species. They consist of several protein groups with different features, such as the overall charge of the molecule, the content of disulphide bonds, and structural stability under environmental stress. Although the three-dimensional structures of several classes of plant peptides are well determined, the mechanism of action of some of these molecules is still not well defined. However, further studies may provide new evidences for their function on bacterial cell wall. Therefore, this paper focuses on plant peptides that show activity against plant-pathogenic and human-pathogenic bacteria. Furthermore, we describe the folding of several peptides and similarities among their three-dimensional structures. Some hypotheses for their mechanisms of action and attack on the bacterial membrane surface are also proposed.
Figures

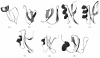
References
-
- Pelegrini PB, Franco OL. Plant γ-thionins: novel insights on the mechanism of action of a multi-functional class of defense proteins. International Journal of Biochemistry and Cell Biology. 2005;37(11):2239–2253. - PubMed
-
- Witkowska D, Bartyś A, Gamian A. Defensins and cathelicidins as natural peptide antibiotics. Postępy Higieny i Medycyny Doświadczalnej. 2008;62:694–707. - PubMed
-
- Daly NL, Rosengren KJ, Craik DJ. Discovery, structure and biological activities of cyclotides. Advanced Drug Delivery Reviews. 2009;61(11):918–930. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

