Osteosarcomagenesis: modeling cancer initiation in the mouse
- PMID: 21403899
- PMCID: PMC3043296
- DOI: 10.1155/2011/694136
Osteosarcomagenesis: modeling cancer initiation in the mouse
Abstract
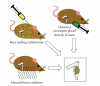
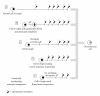
Osteosarcoma remains a deadly malignancy afflicting adolescents and young adults. The lack of a precursor and the panoply of genetic aberrations present in identified osteosarcomas makes study of its initiation difficult. A number of candidate hypotheses have been tested in the mouse, a species with a higher background incidence of osteosarcoma. Chemical carcinogens, external beam radiation, and bone-seeking heavy metal radioisotopes have all proven to be osteosarcomagenic in wild-type mice. A number of oncogenes, introduced via integrating viruses or aberrantly activated from heritable genetic loci, participate in and can individually drive osteosarcomagenesis. Germline and conditional gene ablations in the form of some but not all aneuploidy-inducing genes, conventional tumor suppressors, and factors that function normally in mesenchymal differentiation have also proven osteosarcomagenic, especially in combinations that silence the Rb1 and p53 pathways. This paper reviews the rich history of mouse models of osteosarcomagenesis, what they have taught us about the human disease, and what future mouse experiments yet promise to teach.
Figures

References
-
- Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: national cancer data base report. Clinical Orthopaedics and Related Research. 2007;(459):40–47. - PubMed
-
- Unni KK, Dahlin DC. Osteosarcoma: pathology and classification. Seminars in Roentgenology. 1989;24(3):143–152. - PubMed
-
- Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422–441. - PubMed
-
- Kurt AM, Unni KK, McLeod RA, Pritchard DJ. Low-grade intraosseous osteosarcoma. Cancer. 1990;65(6):1418–1428. - PubMed
-
- Pybus FC, Miller EW. Spontaneous bone tumours of mice. American Journal of Cancer. 1938;33(98):p. 111.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

