Development and validation of transferable amide I vibrational frequency maps for peptides
- PMID: 21405034
- PMCID: PMC3274961
- DOI: 10.1021/jp200745r
Development and validation of transferable amide I vibrational frequency maps for peptides
Abstract
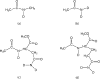
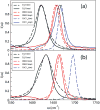
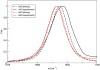
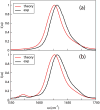
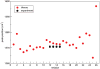
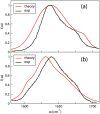
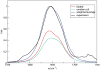
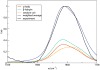
Infrared (IR) spectroscopy of the amide I band has been widely utilized for the analysis of peptides and proteins. Theoretical modeling of IR spectra of proteins requires an accurate and efficient description of the amide I frequencies. In this paper, amide I frequency maps for protein backbone and side chain groups are developed from experimental spectra and vibrational lifetimes of N-methylacetamide and acetamide in different solvents. The frequency maps, along with established nearest-neighbor frequency shift and coupling schemes, are then applied to a variety of peptides in aqueous solution and reproduce experimental spectra well. The frequency maps are designed to be transferable to different environments; therefore, they can be used for heterogeneous systems, such as membrane proteins.
Figures



References
-
- Dickerson RE, Geis I. The structure and action of proteins. W. A. Benjamin, Inc; Menlo Park, CA: 1969.
-
- Nelson DL, Cox MM. Lehninger Principles of Biochemistry. W. H. Freeman and Company; New York: 2005.
-
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. W. H. Freeman and Company; New York: 2007.
-
- McCammon JA, Harvey SC. Dynamics of Proteins and Nucleic Acids. Cambridge University Press; Cambridge: 1987.
-
- Boehr DD, Dyson HJ, Wright PE. Chem Rev. 2006;106:3055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

