Proteases as therapeutics
- PMID: 21406063
- PMCID: PMC4503466
- DOI: 10.1042/BJ20100965
Proteases as therapeutics
Abstract
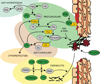
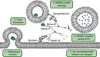
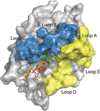
Proteases are an expanding class of drugs that hold great promise. The U.S. FDA (Food and Drug Administration) has approved 12 protease therapies, and a number of next generation or completely new proteases are in clinical development. Although they are a well-recognized class of targets for inhibitors, proteases themselves have not typically been considered as a drug class despite their application in the clinic over the last several decades; initially as plasma fractions and later as purified products. Although the predominant use of proteases has been in treating cardiovascular disease, they are also emerging as useful agents in the treatment of sepsis, digestive disorders, inflammation, cystic fibrosis, retinal disorders, psoriasis and other diseases. In the present review, we outline the history of proteases as therapeutics, provide an overview of their current clinical application, and describe several approaches to improve and expand their clinical application. Undoubtedly, our ability to harness proteolysis for disease treatment will increase with our understanding of protease biology and the molecular mechanisms responsible. New technologies for rationally engineering proteases, as well as improved delivery options, will expand greatly the potential applications of these enzymes. The recognition that proteases are, in fact, an established class of safe and efficacious drugs will stimulate investigation of additional therapeutic applications for these enzymes. Proteases therefore have a bright future as a distinct therapeutic class with diverse clinical applications.
Figures
References
-
- Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta. 2010;1803:39–54. - PubMed
-
- Duffy MJ, McGowan PM, Gallagher WM. Cancer invasion and metastasis: changing views. J. Pathol. 2008;214:283–293. - PubMed
-
- Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat. Rev. Mol. Cell Biol. 2007;8:245–257. - PubMed
-
- Puente XS, Sánchez LM, Gutiérrez-Fernández A, Velasco G, López-Otín C. A genomic view of the complexity of mammalian proteolytic systems. Biochem. Soc. Trans. 2005;33:331–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

