How does early detection by screening affect disease progression? Modeling estimated benefits in prostate cancer screening
- PMID: 21406620
- PMCID: PMC4789305
- DOI: 10.1177/0272989X10396717
How does early detection by screening affect disease progression? Modeling estimated benefits in prostate cancer screening
Abstract
Background: Simulation models are essential tools for estimating benefits of cancer screening programs. Such models include a screening-effect model that represents how early detection by screening followed by treatment affects disease-specific survival. Two commonly used screening-effect models are the stage-shift model, where mortality benefits are explained by the shift to more favorable stages, and the cure model, where early detection enhances the chances of cure from disease.
Objective: This article describes commonly used screening-effect models and analyses their predicted mortality benefit in a model for prostate cancer screening.
Method: The MISCAN simulation model was used to predict the reduction of prostate cancer mortality in the European Randomized Study of Screening for Prostate Cancer (ERSPC) Rotterdam. The screening-effect models were included in the model. For each model the predictions of prostate cancer mortality reduction were calculated. The study compared 4 screening-effect models, which are versions of the stage-shift model or the cure model.
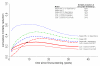
Results: The stage-shift models predicted, after a follow-up of 9 years, reductions in prostate cancer mortality varying from 38% to 63% for ERSPC-Rotterdam compared with a 27% reduction observed in the ERSPC. The cure models predicted reductions in prostate cancer mortality varying from 21% to 27%.
Conclusions: The differences in predicted mortality reductions show the importance of validating models to observed trial mortality data. The stage-shift models considerably overestimated the mortality reduction. Therefore, the stage-shift models should be used with care, especially when modeling the effect of screening for cancers with long lead times, such as prostate cancer.
Figures

Comment in
-
Exploring the unknown and the unknowable with simulation models.Med Decis Making. 2011 Jul-Aug;31(4):521-3. doi: 10.1177/0272989X11412078. Med Decis Making. 2011. PMID: 21757646 No abstract available.
References
-
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2010 Mar-Apr;60(2):99–119. - PubMed
-
- Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001 Nov 21;93(22):1704–13. - PubMed
-
- Benson VS, Patnick J, Davies AK, Nadel MR, Smith RA, Atkin WS. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008 Mar 15;122(6):1357–67. - PubMed
-
- de Gelder R, Bulliard JL, de Wolf C, Fracheboud J, Draisma G, Schopper D, et al. Cost-effectiveness of opportunistic versus organised mammography screening in Switzerland. Eur J Cancer. 2009 Jan;45(1):127–38. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

