Randomised phase II trial of gemcitabine plus vinorelbine vs gemcitabine plus cisplatin vs gemcitabine plus capecitabine in patients with pretreated metastatic breast cancer
- PMID: 21407218
- PMCID: PMC3068513
- DOI: 10.1038/bjc.2011.86
Randomised phase II trial of gemcitabine plus vinorelbine vs gemcitabine plus cisplatin vs gemcitabine plus capecitabine in patients with pretreated metastatic breast cancer
Abstract
Background: An increasing proportion of patients are exposed to anthracyclines and/or taxanes in the adjuvant or neoadjuvant setting. Re-exposure in the metastatic stage is limited by drug resistance, thus evaluation of non-cross-resistant regimens is mandatory.
Methods: Anthracycline-pretreated patients were randomly assigned to three gemcitabine-based regimens. Chemotherapy consisted of gemcitabine 1.000 mg m(-2) plus vinorelbin 25 mg m(-2) on days 1+8 (GemVin), or plus cisplatin 30 mg m(-2) on days 1+8 (GemCis), or plus capecitabine 650 mg m(-2) b.i.d. orally days 1-14 (GemCap), q3w. The primary end point was response rate.
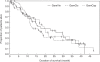
Results: A total of 141 patients were recruited on the trial. The overall response rates were 39.0% (GemVin), 47.7% (GemCis) and 34.7% (GemCap). Median progression-free survival was estimated with 5.7, 6.9 and 8.3 months, respectively. Corresponding median survival times were 17.5 (GemVin), 13.0 (GemCis) and 19.4 months (GemCap). Neutropenia ≥grade 3 occurred in 16.7% (Gem/Vin), 4.4% (GemCis) and 0% (Gem/Cap), whereas non-haematological toxicities were rarely severe except grade 3 hand-foot syndrome in 2.0% of the GemCap patients (per patient analysis).
Conclusions: This randomised phase II trial has revealed comparable results for three gemcitabine-based regimens regarding treatment efficacy and toxicity. Gemcitabine-based chemotherapy appears to be a worthwhile treatment option for pretreated patients with metastatic breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Achanta G, Pelicano H, Feng L, Plunkett W, Huang P (2001) Interaction of p53 and DNA-PK in response to nucleoside analogues: potential role as a sensor complex for DNA damage. Cancer Res 61: 8723–8729 - PubMed
-
- Ajani JA, Welch SR, Raber MN, Fields WS, Krakoff IH (1990) Comprehensive criteria for assessing therapy-induced toxicity. Cancer Invest 8: 147–159 - PubMed
-
- Alauddin A, Shaharyar A (2005) Gemcitabine and cisplatin combination chemotherapy as first-line treatment in patients with metastatic breast cancer. J Clin Oncol, ASCO Annu Meeting Proc (Post-Meeting Edition) 23: abstract 713
-
- Andres R, Mayordomo JI, Lara R, Lastra R, Ortega E, Polo E, Lambea J, Isla D, Saenz-Cusi A, Escudero P, Tres A (2005) Gemcitabine/capecitabine in patients with metastatic breast cancer pretreated with anthracyclines and taxanes. Clin Breast Cancer 6: 158–162 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous