Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration
- PMID: 21407219
- PMCID: PMC3078594
- DOI: 10.1038/bjc.2011.81
Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration
Abstract
Background: Bevacizumab (Bev), a monoclonal antibody to vascular endothelial growth factor (VEGF), is used in combination with chemotherapy for the treatment of metastatic colorectal cancer (CRC). The effects of Bev on angiogenesis have been well described, but the direct effect of Bev on tumour cells is unknown. This study was carried out to determine the molecular and phenotypic changes in CRC cells after chronic Bev exposure in vitro.
Methods: Human CRC cell lines were chronically exposed (3 months) to Bev in vitro to develop Bev-adapted (Bev-A) cell lines. Vascular endothelial growth factor family members were determined by reverse transcription-polymerase chain reaction and western blotting. Migration and invasion was determined using standard in vitro assays. Intravenous injection of tumour cells was carried out to evaluate metastatic potential in mice.
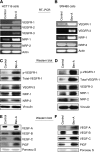
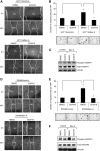
Results: Bevacizumab-adapted cells were found to be more migratory and invasive than control cells (P<0.001). Bevacizumab-adapted cells showed higher levels of VEGF-A, -B, -C, placental growth factor (PlGF), VEGF receptor-1 (VEGFR-1) and phosphorylation of VEGFR-1. Furthermore, treatment with SU5416, a VEGFR protein tyrosine kinase inhibitor, led to significantly decreased cell migration in vitro (P<0.001). Bevacizumab-adapted cells were more metastatic in vivo (P<0.05).
Conclusion: Chronic exposure of CRC cells to Bev (1) increased expression of VEGF-A, -B, -C, PlGF, VEGFR-1 and VEGFR-1 phosphorylation, (2) increased tumour cell migration and invasion, and (3) metastatic potential in vivo. Our study shows the functional significance of autocrine VEGF signalling in CRC cells.
Figures

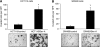

References
-
- Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM, O’Reilly S, Chu L, Azar CA, Lopa S, Wolmark N (2011) Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 29: 11–16 - PMC - PubMed
-
- Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11: 83–95 - PMC - PubMed
-
- Casanovas O, Hicklin DJ, Bergers G, Hanahan D (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8: 299–309 - PubMed
-
- Dallas NA, Gray MJ, Xia L, Fan F, van Buren II G, Gaur P, Samuel S, Lim SJ, Arumugam T, Ramachandran V, Wang H, Ellis LM (2008) Neuropilin-2-mediated tumor growth and angiogenesis in pancreatic adenocarcinoma. Clin Cancer Res 14: 8052–8060 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

