From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells
- PMID: 21407800
- PMCID: PMC3044159
- DOI: 10.1371/journal.pone.0017046
From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells
Abstract
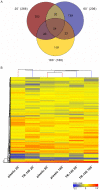
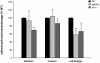
Candida albicans frequently causes superficial infections by invading and damaging epithelial cells, but may also cause systemic infections by penetrating through epithelial barriers. C. albicans is an unusual pathogen because it can invade epithelial cells via two distinct mechanisms: induced endocytosis, analogous to facultative intracellular enteropathogenic bacteria, and active penetration, similar to plant pathogenic fungi. Here we investigated the molecular basis of C. albicans epithelial interactions. By systematically assessing the contributions of defined fungal pathways and factors to different stages of epithelial interactions, we provide an expansive portrait of the processes and activities involved in epithelial infection. We strengthen the concept that hyphal formation is critical for epithelial invasion. Importantly, our data support a model whereby initial epithelial invasion per se does not elicit host damage, but that C. albicans relies on a combination of contact-sensing, directed hyphal extension, active penetration and the expression of novel pathogenicity factors for further inter-epithelial invasion, dissemination and ultimate damage of host cells. Finally, we explore the transcriptional landscape of C. albicans during the early stages of epithelial interaction, and, via genetic analysis, identify ICL1 and PGA34 as novel oral epithelial pathogenicity factors.
Conflict of interest statement
Figures



References
-
- Mavor AL, Thewes S, Hube B. Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr Drug Targets. 2005;6:863–874. - PubMed
-
- Odds FC. Baltimore: University Park Press; 1988. Ecology and epidemiology of candidiasis.
-
- Ruhnke M. Washington DC: American Society for Microbiology Press; 2002. Skin and mucous membrane infections. pp. 307–325.
-
- Calderone R. Washington, DC: ASM Press; 2002. Candida and Candidiasis.
-
- Hube B. From commensal to pathogen: stage- and tissue-specific gene expression of Candida albicans. Curr Opin Microbiol. 2004;7:336–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

