Wild bird migration across the Qinghai-Tibetan plateau: a transmission route for highly pathogenic H5N1
- PMID: 21408010
- PMCID: PMC3052365
- DOI: 10.1371/journal.pone.0017622
Wild bird migration across the Qinghai-Tibetan plateau: a transmission route for highly pathogenic H5N1
Abstract
Background: Qinghai Lake in central China has been at the center of debate on whether wild birds play a role in circulation of highly pathogenic avian influenza virus H5N1. In 2005, an unprecedented epizootic at Qinghai Lake killed more than 6000 migratory birds including over 3000 bar-headed geese (Anser indicus). H5N1 subsequently spread to Europe and Africa, and in following years has re-emerged in wild birds along the Central Asia flyway several times.
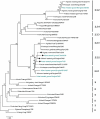
Methodology/principal findings: To better understand the potential involvement of wild birds in the spread of H5N1, we studied the movements of bar-headed geese marked with GPS satellite transmitters at Qinghai Lake in relation to virus outbreaks and disease risk factors. We discovered a previously undocumented migratory pathway between Qinghai Lake and the Lhasa Valley of Tibet where 93% of the 29 marked geese overwintered. From 2003-2009, sixteen outbreaks in poultry or wild birds were confirmed on the Qinghai-Tibet Plateau, and the majority were located within the migratory pathway of the geese. Spatial and temporal concordance between goose movements and three potential H5N1 virus sources (poultry farms, a captive bar-headed goose facility, and H5N1 outbreak locations) indicated ample opportunities existed for virus spillover and infection of migratory geese on the wintering grounds. Their potential as a vector of H5N1 was supported by rapid migration movements of some geese and genetic relatedness of H5N1 virus isolated from geese in Tibet and Qinghai Lake.
Conclusions/significance: This is the first study to compare phylogenetics of the virus with spatial ecology of its host, and the combined results suggest that wild birds play a role in the spread of H5N1 in this region. However, the strength of the evidence would be improved with additional sequences from both poultry and wild birds on the Qinghai-Tibet Plateau where H5N1 has a clear stronghold.
Conflict of interest statement
Figures





References
-
- Guo Y, Xu X, Wan X. Genetic characterization of an avian influenza A (H5N1) virus isolated from a sick goose in China. Zhonghua Shi Xan He Lin Chaung Bing Du Xue Za Zhi. 1998;12:322–325. (in Chinese) - PubMed
-
- Xu XY, Subbarao K, Cox NJ, Guo YJ. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology. 1999;261:15–19. - PubMed
-
- OIE. Update on highly pathogenic avian influenza in animals: Type H5 and H7. OIE website. 2010. Available: http://www.oie.int/downld/AVIAN%20INFLUENZA/A_AI-Asia.htm. Accessed 2010 Oct 5.
-
- Sims LD, Brown IH. Multicontinental epidemic of H5N1 HPAI virus (1996–2007). In: Swayne DE, editor. Avian Influenza. Ames, Iowa, USA: Blackwell Publishing; 2008. pp. 251–286.
-
- Alexander DJ. A review of avian influenza in different bird species. Veterinary Microbiology. 2000;74:3–13. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

