Transcriptome analysis of the vernalization response in barley (Hordeum vulgare) seedlings
- PMID: 21408015
- PMCID: PMC3052371
- DOI: 10.1371/journal.pone.0017900
Transcriptome analysis of the vernalization response in barley (Hordeum vulgare) seedlings
Abstract
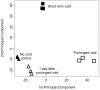
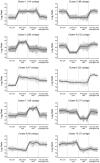
Temperate cereals, such as wheat (Triticum spp.) and barley (Hordeum vulgare), respond to prolonged cold by becoming more tolerant of freezing (cold acclimation) and by becoming competent to flower (vernalization). These responses occur concomitantly during winter, but vernalization continues to influence development during spring. Previous studies identified VERNALIZATION1 (VRN1) as a master regulator of the vernalization response in cereals. The extent to which other genes contribute to this process is unclear. In this study the Barley1 Affymetrix chip was used to assay gene expression in barley seedlings during short or prolonged cold treatment. Gene expression was also assayed in the leaves of plants after prolonged cold treatment, in order to identify genes that show lasting responses to prolonged cold, which might contribute to vernalization-induced flowering. Many genes showed altered expression in response to short or prolonged cold treatment, but these responses differed markedly. A limited number of genes showed lasting responses to prolonged cold treatment. These include genes known to be regulated by vernalization, such as VRN1 and ODDSOC2, and also contigs encoding a calcium binding protein, 23-KD jasmonate induced proteins, an RNase S-like protein, a PR17d secretory protein and a serine acetyltransferase. Some contigs that were up-regulated by short term cold also showed lasting changes in expression after prolonged cold treatment. These include COLD REGULATED 14B (COR14B) and the barley homologue of WHEAT COLD SPECIFIC 19 (WSC19), which were expressed at elevated levels after prolonged cold. Conversely, two C-REPEAT BINDING FACTOR (CBF) genes showed reduced expression after prolonged cold. Overall, these data show that a limited number of barley genes exhibit lasting changes in expression after prolonged cold treatment, highlighting the central role of VRN1 in the vernalization response in cereals.
Conflict of interest statement
Figures




References
-
- Entz MH, Fowler DB. Agronomic performance of winter versus spring wheat. Agronomy Journal. 1991;83:527–532.
-
- King RW, Heide OM. Seasonal flowering and evolution: the heritage from Charles Darwin. Functional Plant Biology. 2009;36:1027–1036. - PubMed
-
- Limin AE, Fowler DB. Low-temperature tolerance and genetic potential in wheat (Triticum aestivum L.): response to photoperiod, vernalization and plant development. Planta. 2006;224:360–366. - PubMed
-
- Koemel JE, Guenzi AC, Anderson JA, Smith EL. Cold hardiness of wheat near-isogenic lines differing in vernalization alleles. Theoretical and Applied Genetics. 2004;109:839–846. - PubMed
-
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:571–599. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

