Changes in the organization of excitation-contraction coupling structures in failing human heart
- PMID: 21408028
- PMCID: PMC3052389
- DOI: 10.1371/journal.pone.0017901
Changes in the organization of excitation-contraction coupling structures in failing human heart
Erratum in
- PLoS One. 2011;6(4). doi:10.1371/annotation/061613ea-0f01-420f-bc3f-af36e5c35790. Ruygrok, Peter R [corrected to Ruygrok, Peter N]
Abstract
Background: The cardiac myocyte t-tubular system ensures rapid, uniform cell activation and several experimental lines of evidence suggest changes in the t-tubular system and associated excitation-contraction coupling proteins may occur in heart failure.
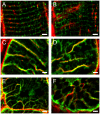
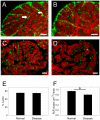
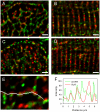
Methods and results: The organization of t-tubules, L-type calcium channels (DHPRs), ryanodine receptors (RyRs) and contractile machinery were examined in fixed ventricular tissue samples from both normal and failing hearts (idiopathic (non-ischemic) dilated cardiomyopathy) using high resolution fluorescent imaging. Wheat germ agglutinin (WGA), Na-Ca exchanger, DHPR and caveolin-3 labels revealed a shift from a predominantly transverse orientation to oblique and axial directions in failing myocytes. In failure, dilation of peripheral t-tubules occurred and a change in the extent of protein glycosylation was evident. There was no change in the fractional area occupied by myofilaments (labeled with phalloidin) but there was a small reduction in the number of RyR clusters per unit area. The general relationship between DHPRs and RyR was not changed and RyR labeling overlapped with 51±3% of DHPR labeling in normal hearts. In longitudinal (but not transverse) sections there was an ∼30% reduction in the degree of colocalization between DHPRs and RyRs as measured by Pearson's correlation coefficient in failing hearts.
Conclusions: The results show that extensive remodelling of the t-tubular network and associated excitation-contraction coupling proteins occurs in failing human heart. These changes may contribute to abnormal calcium handling in heart failure. The general organization of the t-system and changes observed in failure samples have subtle differences to some animal models although the general direction of changes are generally similar.
Conflict of interest statement
Figures
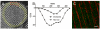


References
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Cannell MB, Soeller C. Sparks of interest in cardiac excitation-contraction coupling. Trends Pharmacol Sci. 1998;19:16–20. - PubMed
-
- Franzini-Armstrong C. Functional implications of RyR-dHPR relationships in skeletal and cardiac muscles. Biol Res. 2004;37:507–512. - PubMed
-
- Cheng H, Cannell MB, Lederer WJ. Propagation of excitation-contraction coupling into ventricular myocytes. Pflugers Arch. 1994;428:415–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

