An atlas for Schistosoma mansoni organs and life-cycle stages using cell type-specific markers and confocal microscopy
- PMID: 21408085
- PMCID: PMC3050934
- DOI: 10.1371/journal.pntd.0001009
An atlas for Schistosoma mansoni organs and life-cycle stages using cell type-specific markers and confocal microscopy
Abstract
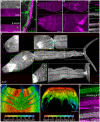
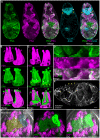
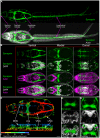
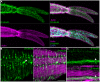
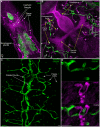
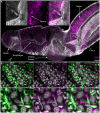
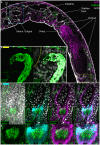
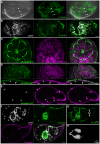
Schistosomiasis (bilharzia) is a tropical disease caused by trematode parasites (Schistosoma) that affects hundreds of millions of people in the developing world. Currently only a single drug (praziquantel) is available to treat this disease, highlighting the importance of developing new techniques to study Schistosoma. While molecular advances, including RNA interference and the availability of complete genome sequences for two Schistosoma species, will help to revolutionize studies of these animals, an array of tools for visualizing the consequences of experimental perturbations on tissue integrity and development needs to be made widely available. To this end, we screened a battery of commercially available stains, antibodies and fluorescently labeled lectins, many of which have not been described previously for analyzing schistosomes, for their ability to label various cell and tissue types in the cercarial stage of S. mansoni. This analysis uncovered more than 20 new markers that label most cercarial tissues, including the tegument, the musculature, the protonephridia, the secretory system and the nervous system. Using these markers we present a high-resolution visual depiction of cercarial anatomy. Examining the effectiveness of a subset of these markers in S. mansoni adults and miracidia, we demonstrate the value of these tools for labeling tissues in a variety of life-cycle stages. The methodologies described here will facilitate functional analyses aimed at understanding fundamental biological processes in these parasites.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Basch PF. New York: Oxford University Press; 1991. Schistosomes: Development, Reproduction, and Host Relations.248
-
- Skelly PJ, Da'dara A, Harn DA. Suppression of cathepsin B expression in Schistosoma mansoni by RNA interference. Int J Parasitol. 2003;33:363–369. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

