Trypanosoma cruzi utilizes the host low density lipoprotein receptor in invasion
- PMID: 21408103
- PMCID: PMC3051337
- DOI: 10.1371/journal.pntd.0000953
Trypanosoma cruzi utilizes the host low density lipoprotein receptor in invasion
Abstract
Background: Trypanosoma cruzi, an intracellular protozoan parasite that infects humans and other mammalian hosts, is the etiologic agent in Chagas disease. This parasite can invade a wide variety of mammalian cells. The mechanism(s) by which T. cruzi invades its host cell is not completely understood. The activation of many signaling receptors during invasion has been reported; however, the exact mechanism by which parasites cross the host cell membrane barrier and trigger fusion of the parasitophorous vacuole with lysosomes is not understood.
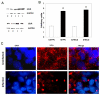
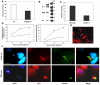
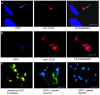
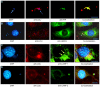
Methodology/principal findings: In order to explore the role of the Low Density Lipoprotein receptor (LDLr) in T. cruzi invasion, we evaluated LDLr parasite interactions using immunoblot and immunofluorescence (IFA) techniques. These experiments demonstrated that T. cruzi infection increases LDLr levels in infected host cells, inhibition or disruption of LDLr reduces parasite load in infected cells, T. cruzi directly binds recombinant LDLr, and LDLr-dependent T. cruzi invasion requires PIP2/3. qPCR analysis demonstrated a massive increase in LDLr mRNA (8000 fold) in the heart of T. cruzi infected mice, which is observed as early as 15 days after infection. IFA shows a co-localization of both LDL and LDLr with parasites in infected heart.
Conclusions/significance: These data highlight, for the first time, that LDLr is involved in host cell invasion by this parasite and the subsequent fusion of the parasitophorous vacuole with the host cell lysosomal compartment. The model suggested by this study unifies previous models of host cell invasion for this pathogenic protozoon. Overall, these data indicate that T. cruzi targets LDLr and its family members during invasion. Binding to LDL likely facilitates parasite entry into host cells. The observations in this report suggest that therapeutic strategies based on the interaction of T. cruzi and the LDLr pathway should be pursued as possible targets to modify the pathogenesis of disease following infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Membrane cholesterol regulates lysosome-plasma membrane fusion events and modulates Trypanosoma cruzi invasion of host cells.PLoS Negl Trop Dis. 2012;6(3):e1583. doi: 10.1371/journal.pntd.0001583. Epub 2012 Mar 27. PLoS Negl Trop Dis. 2012. PMID: 22479662 Free PMC article.
-
Host cell invasion and oral infection by Trypanosoma cruzi strains of genetic groups TcI and TcIV from chagasic patients.Parasit Vectors. 2016 Apr 1;9:189. doi: 10.1186/s13071-016-1455-z. Parasit Vectors. 2016. PMID: 27038796 Free PMC article.
-
Mechanisms of host cell invasion by Trypanosoma cruzi.Adv Parasitol. 2011;76:33-61. doi: 10.1016/B978-0-12-385895-5.00002-5. Adv Parasitol. 2011. PMID: 21884886 Review.
-
The host Rab9a/Rab32 axis is actively recruited to the Trypanosoma cruzi parasitophorous vacuole and benefits the infection cycle.Mol Microbiol. 2024 Nov;122(5):643-659. doi: 10.1111/mmi.15217. Epub 2024 Jan 9. Mol Microbiol. 2024. PMID: 38193389
-
The role of host cell lysosomes in Trypanosoma cruzi invasion.Subcell Biochem. 2008;47:165-73. doi: 10.1007/978-0-387-78267-6_13. Subcell Biochem. 2008. PMID: 18512350 Review.
Cited by
-
Metabolic syndrome: an ill wind that blows some good?Diabetes Metab Res Rev. 2015 May;31(4):344-5. doi: 10.1002/dmrr.2635. Epub 2015 Feb 23. Diabetes Metab Res Rev. 2015. PMID: 25611014 Free PMC article.
-
Metabolic syndrome agravates cardiovascular, oxidative and inflammatory dysfunction during the acute phase of Trypanosoma cruzi infection in mice.Sci Rep. 2019 Dec 11;9(1):18885. doi: 10.1038/s41598-019-55363-9. Sci Rep. 2019. PMID: 31827186 Free PMC article.
-
Trypanosoma cruzi infection and host lipid metabolism.Mediators Inflamm. 2014;2014:902038. doi: 10.1155/2014/902038. Epub 2014 Sep 3. Mediators Inflamm. 2014. PMID: 25276058 Free PMC article. Review.
-
Curcumin Enhances the Anti-Trypanosoma cruzi Activity of Benznidazole-Based Chemotherapy in Acute Experimental Chagas Disease.Antimicrob Agents Chemother. 2016 May 23;60(6):3355-64. doi: 10.1128/AAC.00343-16. Print 2016 Jun. Antimicrob Agents Chemother. 2016. PMID: 27001816 Free PMC article.
-
The brighter (and evolutionarily older) face of the metabolic syndrome: evidence from Trypanosoma cruzi infection in CD-1 mice.Diabetes Metab Res Rev. 2015 May;31(4):346-359. doi: 10.1002/dmrr.2636. Epub 2015 Mar 6. Diabetes Metab Res Rev. 2015. PMID: 25613819 Free PMC article.
References
-
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
-
- Sudhof TC, Russel DW, Goldstein JL, Brown MS. Casette of eight exons shared by genes for LDL receptor and EGF pecursor. Science. 1985;228:893–95. - PubMed
-
- Chen WJ, Goldstein L, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

