A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion
- PMID: 21408136
- PMCID: PMC3050925
- DOI: 10.1371/journal.pone.0017649
A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion
Abstract
Background: Extracellular Hsp90 protein (eHsp90) potentiates cancer cell motility and invasion through a poorly understood mechanism involving ligand mediated function with its cognate receptor LRP1. Glioblastoma multiforme (GBM) represents one of the most aggressive and lethal brain cancers. The receptor tyrosine kinase EphA2 is overexpressed in the majority of GBM specimens and is a critical mediator of GBM invasiveness through its AKT dependent activation of EphA2 at S897 (P-EphA2(S897)). We explored whether eHsp90 may confer invasive properties to GBM via regulation of EphA2 mediated signaling.
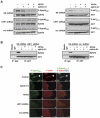
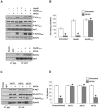
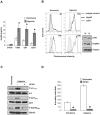
Principal findings: We find that eHsp90 signaling is essential for sustaining AKT activation, P-EphA2(S897), lamellipodia formation, and concomitant GBM cell motility and invasion. Furthermore, eHsp90 promotes the recruitment of LRP1 to EphA2 in an AKT dependent manner. A finding supported by biochemical methodology and the dual expression of LRP1 and P-EphA2(S897) in primary and recurrent GBM tumor specimens. Moreover, hypoxia mediated facilitation of GBM motility and invasion is dependent upon eHsp90-LRP1 signaling. Hypoxia dramatically elevated surface expression of both eHsp90 and LRP1, concomitant with eHsp90 dependent activation of src, AKT, and EphA2.
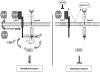
Significance: We herein demonstrate a novel crosstalk mechanism involving eHsp90-LRP1 dependent regulation of EphA2 function. We highlight a dual role for eHsp90 in transducing signaling via LRP1, and in facilitating LRP1 co-receptor function for EphA2. Taken together, our results demonstrate activation of the eHsp90-LRP1 signaling axis as an obligate step in the initiation and maintenance of AKT signaling and EphA2 activation, thereby implicating this pathway as an integral component contributing to the aggressive nature of GBM.
Conflict of interest statement
Figures


References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Drappatz J, Norden AD, Wen PY. Therapeutic strategies for inhibiting invasion in glioblastoma. Expert Rev Neurother. 2009;9:519–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

