New modularity of DAP-kinases: alternative splicing of the DRP-1 gene produces a ZIPk-like isoform
- PMID: 21408167
- PMCID: PMC3050894
- DOI: 10.1371/journal.pone.0017344
New modularity of DAP-kinases: alternative splicing of the DRP-1 gene produces a ZIPk-like isoform
Abstract
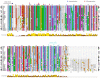
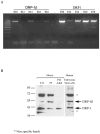
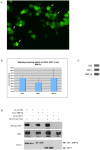
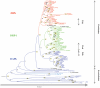
DRP-1 and ZIPk are two members of the Death Associated Protein Ser/Thr Kinase (DAP-kinase) family, which function in different settings of cell death including autophagy. DAP kinases are very similar in their catalytic domains but differ substantially in their extra-catalytic domains. This difference is crucial for the significantly different modes of regulation and function among DAP kinases. Here we report the identification of a novel alternatively spliced kinase isoform of the DRP-1 gene, termed DRP-1β. The alternative splicing event replaces the whole extra catalytic domain of DRP-1 with a single coding exon that is closely related to the sequence of the extra catalytic domain of ZIPk. As a consequence, DRP-1β lacks the calmodulin regulatory domain of DRP-1, and instead contains a leucine zipper-like motif similar to the protein binding region of ZIPk. Several functional assays proved that this new isoform retained the biochemical and cellular properties that are common to DRP-1 and ZIPk, including myosin light chain phosphorylation, and activation of membrane blebbing and autophagy. In addition, DRP-1β also acquired binding to the ATF4 transcription factor, a feature characteristic of ZIPk but not DRP-1. Thus, a splicing event of the DRP-1 produces a ZIPk like isoform. DRP-1β is highly conserved in evolution, present in all known vertebrate DRP-1 loci. We detected the corresponding mRNA and protein in embryonic mouse brains and in human embryonic stem cells thus confirming the in vivo utilization of this isoform. The discovery of module conservation within the DAPk family members illustrates a parsimonious way to increase the functional complexity within protein families. It also provides crucial data for modeling the expansion and evolution of DAP kinase proteins within vertebrates, suggesting that DRP-1 and ZIPk most likely evolved from their ancient ancestor gene DAPk by two gene duplication events that occurred close to the emergence of vertebrates.
Conflict of interest statement
Figures




References
-
- Bialik S, Kimchi A. The death-associated protein kinases: structure, function, and beyond. Annu Rev Biochem. 2006;75:189–210. - PubMed
-
- Shohat G, Shani G, Eisenstein M, Kimchi A. The DAP-kinase family of proteins: study of a novel group of calcium-regulated death-promoting kinases. Biochim Biophys Acta. 2002;1600:45–50. - PubMed
-
- Bialik S, Kimchi A. DAP-kinase as a target for drug design in cancer and diseases associated with accelerated cell death. Semin Cancer Biol. 2004;14:283–294. - PubMed
-
- Esteller M. Relevance of DNA methylation in the management of cancer. Lancet Oncol. 2003;4:351–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

