Host reproductive phenology drives seasonal patterns of host use in mosquitoes
- PMID: 21408172
- PMCID: PMC3049777
- DOI: 10.1371/journal.pone.0017681
Host reproductive phenology drives seasonal patterns of host use in mosquitoes
Abstract
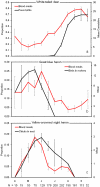
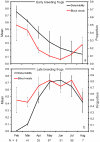
Seasonal shifts in host use by mosquitoes from birds to mammals drive the timing and intensity of annual epidemics of mosquito-borne viruses, such as West Nile virus, in North America. The biological mechanism underlying these shifts has been a matter of debate, with hypotheses falling into two camps: (1) the shift is driven by changes in host abundance, or (2) the shift is driven by seasonal changes in the foraging behavior of mosquitoes. Here we explored the idea that seasonal changes in host use by mosquitoes are driven by temporal patterns of host reproduction. We investigated the relationship between seasonal patterns of host use by mosquitoes and host reproductive phenology by examining a seven-year dataset of blood meal identifications from a site in Tuskegee National Forest, Alabama USA and data on reproduction from the most commonly utilized endothermic (white-tailed deer, great blue heron, yellow-crowned night heron) and ectothermic (frogs) hosts. Our analysis revealed that feeding on each host peaked during periods of reproductive activity. Specifically, mosquitoes utilized herons in the spring and early summer, during periods of peak nest occupancy, whereas deer were fed upon most during the late summer and fall, the period corresponding to the peak in births for deer. For frogs, however, feeding on early- and late-season breeders paralleled peaks in male vocalization. We demonstrate for the first time that seasonal patterns of host use by mosquitoes track the reproductive phenology of the hosts. Peaks in relative mosquito feeding on each host during reproductive phases are likely the result of increased tolerance and decreased vigilance to attacking mosquitoes by nestlings and brooding adults (avian hosts), quiescent young (avian and mammalian hosts), and mate-seeking males (frogs).
Conflict of interest statement
Figures
References
-
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;44:e82. doi: 10.1371/journal.pbio.0040082. - DOI - PMC - PubMed
-
- Dalrymple JM, Young OP, Eldridge BF, Russell PK. Ecology of arboviruses in a Maryland freshwater swamp: III. Vertebrate hosts. Am J Epidemiol. 1972;96:129–140. - PubMed
-
- Edman JD, Taylor DJ. Culex nigripalpus: Seasonal shift in the bird-mammal feeding ratio in a mosquito vector of human encephalitis. Science. 1968;161:67–68. - PubMed
-
- Weaver S, Barrett A. Transmission cycles, host range, evolution and emergence of arboviral disease. Nature Rev Micro 2. 2004;10:789–801. doi: 10.1038/nrmicro1006. - DOI - PMC - PubMed
-
- Edman JD. Host-feeding patterns of Florida mosquitoes III. Culex (Culex) and Culex (Neoculex). J Med Ent. 1974;11:95–104. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

