Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging
- PMID: 21408175
- PMCID: PMC3049780
- DOI: 10.1371/journal.pone.0017487
Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging
Abstract
Background: Accumulation of DNA damage leading to adult stem cell exhaustion has been proposed to be a principal mechanism of aging. Here we tested this hypothesis in healthy individuals of different ages by examining unrepaired DNA double-strand breaks (DSBs) in hematopoietic stem/progenitor cells matured in their physiological microenvironment.
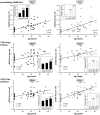
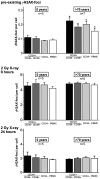
Methodology/principal findings: To asses DNA damage accumulation and repair capacities, γH2AX-foci were examined before and after exposure to ionizing irradiation. Analyzing CD34+ and CD34- stem/progenitor cells we observed an increase of endogenous γH2AX-foci levels with advancing donor age, associated with an age-related decline in telomere length. Using combined immunofluorescence and telomere-fluorescence in-situ hybridization we show that γH2AX-foci co-localize consistently with other repair factors such as pATM, MDC1 and 53BP1, but not significantly with telomeres, strongly supporting the telomere-independent origin for the majority of foci. The highest inter-individual variations for non-telomeric DNA damage were observed in middle-aged donors, whereas the individual DSB repair capacity appears to determine the extent of DNA damage accrual. However, analyzing different stem/progenitor subpopulations obtained from healthy elderly (>70 years), we observed an only modest increase in DNA damage accrual, most pronounced in the primitive CD34+CD38(-)-enriched subfraction, but sustained DNA repair efficiencies, suggesting that healthy lifestyle may slow down the natural aging process.
Conclusions/significance: Based on these findings we conclude that age-related non-telomeric DNA damage accrual accompanies physiological stem cell aging in humans. Moreover, aging may alter the functional capacity of human stem cells to repair DSBs, thereby deteriorating an important genome protection mechanism leading to exceeding DNA damage accumulation. However, the great inter-individual variations in middle-aged individuals suggest that additional cell-intrinsic mechanisms and/or extrinsic factors contribute to the age-associated DNA damage accumulation.
Conflict of interest statement
Figures


References
-
- Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. - PubMed
-
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. - PubMed
-
- Rube CE, Dong X, Kuhne M, Fricke A, Kaestner L, et al. DNA double-strand break rejoining in complex normal tissues. Int J Radiat Oncol Biol Phys. 2008;72:1180–1187. - PubMed
-
- Rube CE, Grudzenski S, Kuhne M, Dong X, Rief N, et al. DNA double-strand break repair of blood lymphocytes and normal tissues analysed in a preclinical mouse model: implications for radiosensitivity testing. Clin Cancer Res. 2008;14:6546–6555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

